Research
Research Goals of the Rongxiang Xu Center...
Our research aims to advance regenerative therapeutics in diabetic wound healing.
Please see below to learn about some of our ongoing research.
Predictive and Diagnostic Biomarkers for Diabetic Foot Ulcers: Open Wound Master Study (DFC)

Initiated in December 2022, the Diabetic Foot Consortium (DFC) is supported by the NIH’s National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Here at BIDMC & Harvard Medical School, we conduct clinical work to support the ambitious aspirations of the DFC. Please visit their website, diabeticfootconsortium.org, to learn more about the DFC and the outcomes it hopes to achieve.
Diabetic foot ulceration is a devastating complication of diabetes that compromises the quality of life, increases risks for lower extremity amputations and further increases risk of death. The main aim is to develop a proteomics biomarker signature prognostic for diabetic wound healing and initiate the determination of its clinical utility. These advances will offer long-term potential for accelerating prognostication tools and biomarker-guided drug development strategies in this field.
This is a standardized observational cohort study that aims to assess the healing time, standard of care (SOC), biomarkers, and other properties of diabetic foot ulcers (DFUs). Research staff at BIDMC plan to collect biosamples from patients with DFUs to assess the biomarkers present in each sample. Biosamples include but are not limited to DFU tissue, blood, urine, and primary wound dressings. Biosamples will be sent to CLASS laboratory for processing and analysis. All data from biosamples will comprise a biorepository for concurrent and future research; some excess biosamples may also go to an NIDDK Central Repository.
Development of mRNA-LNPs delivery system for healing-associated targets in diabetic wounds
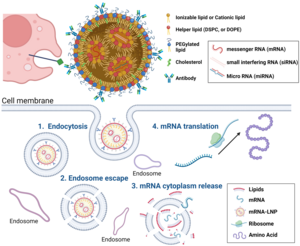
Recently, we have shown that chronic inflammation is significantly associated with failure to heal DFUs. Our research has identified certain genes that are upregulated in a unique cell population observed in patients with healing DFUs. This unique cell population overexpresses inflammatory genes to convert the chronic inflammation of DFUs to an acute inflammatory response. Thus, we are in the process of formulating a controlled modified-mRNA lipid nanoparticle (LNPs) delivery bandage that delivers the genes of interest to enhance gene expression within the wound base.
Strain-Programmable Bioadhesive Patch for Accelerated Healing of Diabetic Ulcer
 Our team recently developed a novel tissue adhesive in the form of a dry double-sided tape (DST) made from a combination of polymer (e.g., chitosan) and crosslinked poly(acrylic acid) grafted with NHS ester to address shortcomings of the existing tissue adhesives and wound dressings including weak and unstable adhesion on wet or wounded tissues and organs. The adhesion mechanism of the DST relies on the removal of interfacial water from the tissue surface, resulting in fast temporary crosslinking to the tissue surface within five seconds. Subsequently, covalent crosslinking with amine groups on the tissue surface then further improves adhesion stability and strength. This unique mechanism allows the DST to form instant yet robust adhesion on wet or wounded tissues that have not been possible by existing tissue adhesive and wound dressings.
Our team recently developed a novel tissue adhesive in the form of a dry double-sided tape (DST) made from a combination of polymer (e.g., chitosan) and crosslinked poly(acrylic acid) grafted with NHS ester to address shortcomings of the existing tissue adhesives and wound dressings including weak and unstable adhesion on wet or wounded tissues and organs. The adhesion mechanism of the DST relies on the removal of interfacial water from the tissue surface, resulting in fast temporary crosslinking to the tissue surface within five seconds. Subsequently, covalent crosslinking with amine groups on the tissue surface then further improves adhesion stability and strength. This unique mechanism allows the DST to form instant yet robust adhesion on wet or wounded tissues that have not been possible by existing tissue adhesive and wound dressings.
Building upon our recent advances, we propose to develop a strain-programmed bioadhesive patch by combining the DST with a novel hydration-based shape memory method. The resultant strain-programmed bioadhesive patch takes the form of thin patch consisting of a non-adhesive elastomer backing and a bioadhesive layer made of the DST. Once hydrated by the native physiological fluids or moisture from the wounded tissue, the patch instantly and robustly adheres to the underlying tissue based on the DST while releasing its programmed strain to provide mechanical contraction to the wound.
Our current work focuses on the development of a novel, proprietary biomaterial that will be very effective in promoting wound healing in experimental diabetes and ex vivo human diabetic skin experiments. Given the lack of any satisfactory treatments for DFU, successful completion of the proposed experiments will lead to new therapeutic approaches in a condition associated with high morbidity and mortality and with serious unmet needs.
Exploring the Effect of Repurposed Drug on Wound Healing
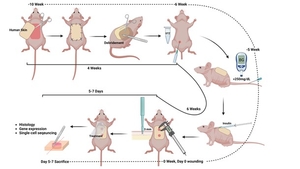
Certain genes are upregulated in a unique cell population observed in patients with healing DFUs. We have identified a repurposed drug that may induce related gene expression in DFU patients with healing wounds.
We developed and optimized a drug delivery platform by loading the repurposed drug into alginate macro porous hydrogels to achieve sustained release rate. The therapeutic potential of the repurposed drug was first validated in various diabetic animal models. The mechanisms of the repurposed drug and the important pathways that are involved in diabetic wound healing will be further investigated by using single-cell genomic technologies.
Diabetic Lower Extremity Discarded Specimens
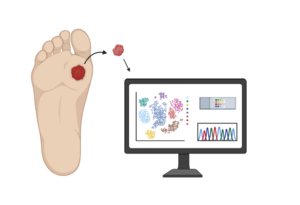
Initiated in 2019, this study assesses transcriptomic (RNA) and proteomic (protein) changes in single cells of the skin of diabetic patients with or without foot ulceration. A combined understanding of single cell transcriptome and proteome levels has the potential to greatly enhance our understanding in an agnostic way regarding the interaction of individual cells in the expression of various genes and production of proteins associated with wound healing. We propose to use an innovative CITE-seq approach that combines highly multiplexed protein marker detection with unbiased transcriptome profiling from foot skin biopsies from healthy, non-DM subjects and DM patients with healed and non-healed DFU. Our main aim is to evaluate differences in gene expression in various cell types and how this translate to expression of specific protein markers. We hypothesize that diabetic patients with impaired wound healing will have aberrant gene and protein expression that will lead to a chronic inflammation stage that precludes linear progression to the next phases of wound healing.
Laboratory Collaborations
We would like to express our gratitude to:
Dr. David Mooney, head of the Mooney Lab at the Wyss Institute for Biologically Inspired Engineering at the Harvard John A. Paulson School of Engineering.
Dr. Monika Niewczas, Joslin Diabetes Center & Harvard Medical School.
Dr. Jonathan Garlick, head of the Garlick Lab at Tufts University School of Dental Medicine.
Dr. Xuanhe Zhao, head of the Zhao Lab at Massachusetts Institute of Technology.
Dr. Parag Chitnis, George Mason University
Dr. Amay Bandodkar, North Carolina State University
Dr. Tengfei Ma, Stony Brook University
SanaHeal, Bioadhesive Technology and Engineering Company.
