Fluorescent Activated Cell Sorters

Beckman Coulter MoFlo AstriosEQ: A high-speed cell sorter (up to 70,000 cells/second) and analyzer (up to 100,000 cells/second), with four fixed lasers (405nm, 488nm, 561nm, and 640nm). The AstriosEQ has the capacity to acquire 28 parameters simultaneously. In addition, it contains two forward scatter parameters and three side scatter parameters, allowing for analysis of extracellular vesicles. Two-, Four-, and Six-way sorting is available. Sorting can be done into tubes of various sizes, plates and custom slides. Single cell sorting into plates is also available.
Operating software for the MoFlo AstriosEQ is the Beckman Coulter Summit program, version 6.3. Operation of this instrument requires a dedicated technician to align lasers, maintain the instrument and to assure its reliable and effective utilization.

BD FACSymphony™ S6: A special order five laser cell sorters (up to 22,000 cells/second) and analyzer (up to 70,000 cells/second) equipped with a solid state 50mW 488nm laser, JDS Uniphase 40mW 640nm HeNe laser, Coherent Compass 40mW 561nm laser (allowing for the acquisition, analysis and sorting of a variety of fluorescent proteins), Lightwave Mode Lock 60mW 355nm UV laser, and Coherent VioFlame 50mW 405nm violet laser. Besides the forward and side scatter parameters, the instrument is equipped for detection of four fluorescent parameters from the 488nm laser, three parameters from the 640nm laser, four parameters from the 561nm laser, six parameters from the violet laser and six parameters from the UV laser. Sorting can be done into tubes of various sizes, plates and custom slides. Single cell sorting into plates is also available.
Operating software for the S6 is the Becton Dickinson Diva software, version 9.5.1, operating on an HP xw4600 Core 2 duo. Operation of this instrument requires a dedicated technician to maintain the instrument and to assure its reliable and effective utilization.

Beckman Coulter Cytoflex SRT: A benchtop cell sorter with four lasers (405nm, 488nm, 561nm and 640nm) and up to 15 fluorescent detectors simultaneously. It contains a violet side scatter available allowing analysis and sort of small particles. Besides the forward and sice scatter paraments, the instrument has two fluorescent channels from 488 nm (Blue) laser, five channels from 561nm (Yellow Green) laser, three channels from 638 nm (Red) laser and five channels from 405 nm (Violet) laser. The sorting can be performed into 15 mL conical tubes, 5 mL FACS tubes, 2 or 1.5 mL eppendorfs tubes, 24, 48 and 96 well plates.
The operating software for the Cytoflex SRT is the Beckman Coulter CytExpert SRT program, version 1.2.0. Operation of this instrument requires a dedicated technician to maintain the instrument and to assure its reliable and effective utilization.
FLOW CYTOMETRY ANALYZERS

Cytek Aurora: A spectral flow cytometer that has the 4 laser system (16V-14B-10YG-8R) which offers up to 40 fluorescent parameters. The Aurora has state-of-the-art optics that allow for excellent sensitivity and resolution without needing filter changes. The flat top beam profile lasers are 405 nm is 100mW (violet detector), 488nm is 50mW (blue detector), 561nm is 50mW (yellow-green detector), and the 640nm is 80mW (red detector). The FSC is based off the 488nm with two SSC, one VSSC off the 405nm and the other BSSC off the 488nm. Sampling rates are low 15uL/min, medium 30 uL/min, high 60uL/min and a plate high throughput at 100 uL/min. Does require a separate end analysis program (FlowJo, FCSexpress, etc.). This machine excels in high complexity panels for rare and dim populations. Please make sure on the weekends that QC has been run before doing your experiment.
|
Violet detector module |
16 channels unevenly spaced bandwidth from 420-829 nm |
|
Blue detector module |
14 channels unevenly spaced bandwidth from 498-829 nm |
|
Yellow-Green detector module |
10 channels unevenly spaced bandwidth from 567-829 nm |
|
Red detector module |
8 channels unevenly spaced bandwidth from 652-829 nm |
The operating software for Aurora is the Cytek Spectro Flo program, version 3.3.0. Operation of this instrument and software requires training from the Flow Cytometry Core Staff.

Nexcelom Celigo: The Celigo adherent cell cytometer allows in situ brightfield and fluorescent analysis of adherent cells. Cell number, density, area density, diameter, area, and perimeter can all be measured. Using LEDs, the system can obtain data for three fluorescent channels (ex 377nm/em 447nm, ex 483nm/em 536nm, and ex 531nm/em 629nm) and 1 brightfield channel. 384, 96, 24, 12, 6, and 1536 well plates can be analyzed; as well as T-25 and T-75 flasks.
The software for acquisition and analysis is the Nexcelom Celigo software version 1.3, running on a Dell Precision T3500. Training from the Flow Cytometry Core staff is required for the users interested in using and operating the instrument/software. It is available to trained users for full use even in off hours, including evenings, nights, and weekends. Access to the instrument during off hours is controlled by a coded electronic card access system, which limits access to approved users only.

Two Beckman Coulter CytoFLEX LX: The instrument has the capacity for 23 parameters, including 21 for fluorescence detection. The fully activated instrument includes three fluorescent channels from the 375 nm (NUV) laser, five from the 405 nm (Violet) laser, three from the 488 nm (Blue) laser, five from the 561 nm (Yellow Green) laser, three from the 638 nm (Red) laser, and two from the 808 nm (Infrared) laser. Option to configure Avalanche Photo Diode detector array to collect side scatter signal from Violet (405 nm) laser. The configured channel (VSSC) can be used to better resolve nanoparticles. 96-well Standard Flat, U and V bottom plates.
Operating software for the CytoFLEX LX is the Beckman Coulter CytExpert program, version 2.6.1 Operation of this instrument requires training by the Flow Core faculty. CytExpert software is available for free download only on a Windows system or Virtual PC.

Beckman Coulter CytoFLEX Nano: The instrument is designed to analyze nanoparticles, at least as small as 40 nm. It offers 4 lasers (405nm, 488nm, 561nm and 638nm) and 5 side scatters channels, specially 2 side scatters on violet laser giving more sensitivity for the detection of small particles. Besides that, the instrument includes one fluorescent channel from 405 nm (Violet) laser, one from 488nm (Blue) laser, one from 561 nm (Yellow Green) laser and three from 638nm (Red) laser. The samples can be acquired from 5 mL tubes and 1.5/2 mL Eppendorf tubes.
The operating software for the CytoFLEX Nano is the Beckman Coulter CytExpert Nano program, version 1.0.0.627. The operation of this instrument is only performed by one of the flow core staff members and requires booking in advance.
Analysis Software
- FlowJo: FlowJo software reads flow cytometry data and facilitates complex data analysis in a graphically intuitive way. FlowJo licenses can be purchased through the Flow Cytometry Core for a fee of $270/year (subject to change).
- FCS Express: FCS Express software reads flow cytometry data and facilitates complex data analysis in a graphically intuitive way.
- Beckman Coulter Kaluza: Kaluza Software challenges today’s solutions with a new vision of what flow cytometry analysis should be. Kaluza provides visualization tools, speed and innovative simplicity to the flow community. With features designed to make your life easier in the lab, Kaluza aims to provide a greater understanding of the data generated by modern cytometers. Kaluza employs patent pending technology that allows for real time analysis of high content files allowing you to spend your valuable time on discovery.
- CytExpert: Software for the Beckman Coulter CytoFlex data only. CytExpert software is available for free download. It only runs on a Window system.
- FCMPass: Software for single extracellular vesicle flow cytometry calibration and reporting data. Available for Windows and MAC system. It is only performed by one of flow cytometry core staff members.
- MPAPass: Software for extracellular vesicle analysis of Miltenyi MACSPlex Exosome kits along with custom multiplex arrays. Available for Windows and MAC system. It is only performed by one of flow cytometry core staff members.
There is one first come, first serve basis for a free analysis computer in the core facility that has no sign-up times or usage fees. Please notify the staff if the computer has any issues. DO NOT leave any data on this computer. Data will be dumped constantly.
MassNano nanoparticle characterization facility

SPARTA BIODISCOVERY AGIS-1: Label-free single particle optical tweezer trapping with Raman Spectroscopy analysis for chemical composition and dynamic reaction monitoring. Allows for chemical formulations of LNPs for production.

UNCHAINED LABS LEPRECHAUN: Interferometer and immunofluorescence for titer, structure, RNA content, and contaminant
analysis of Lentivirus, viral vectors, and exosomes.

UNCHAINED LABS STUNNER: UV/Vis and dynamic light scattering for LNP sizing and polydispersity, concentration, aggregation, and RNA quantification. Nanovesicle protein quantification and conjugate characterization. Viral vector capsid titers and content ratios.

Spectradyne ARC: Microfluidic pulse sensing in a non-optical fluorescent array. Size and concentration via non-laser driven pulse resistance voltage measurements.
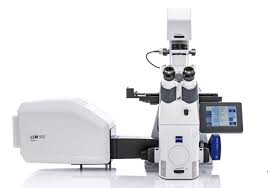
ZEISS LSM 900 + Axiovert: Confocal microscope with Airyscan 2 and Dynamics profiler for resolution of 90 nm with Fluorescent Correlation Spectroscopy to understand population dynamics and molecular concentration. Allows for FRET and FRAP for greater dye pairings and cellular interactions. Includes also the Zen Advanced Dynamics Profiler with Zen Toolkit Molecular quantification.
40x and 100x phase contrast optics.
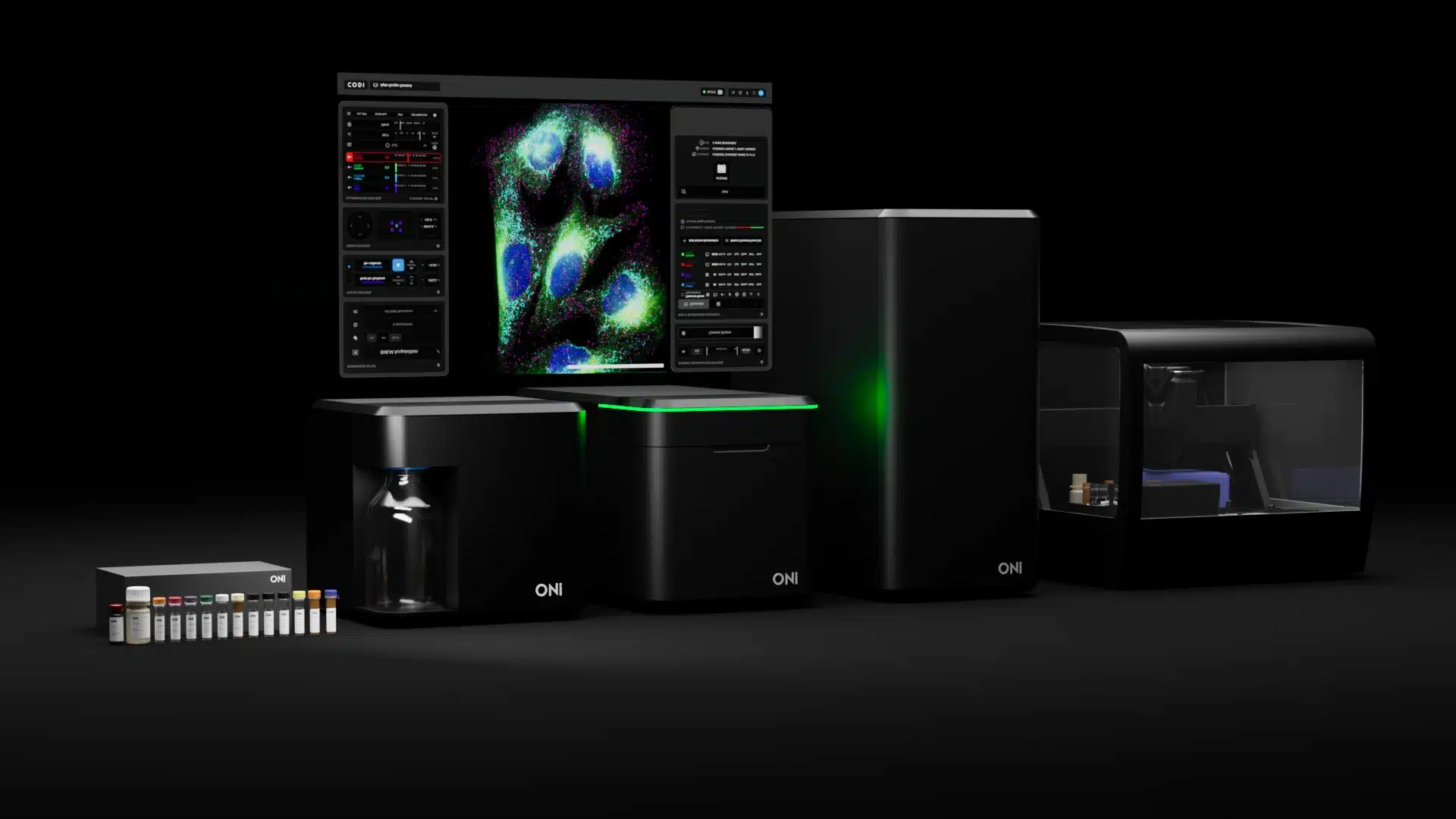
ONi APLOSCOPE: Super resolution SMLM with 15 nm resolution. Capable of single molecule tracking, live cell imaging, protein interactions, and dSTORM/PALM. Includes the APLOFLOW, an automated fluidics system and sample preparation.

PARTICLE METRIX ZetaView Evolution QUATT: Laser NTA for size, concentration, and ZetaPotential. In addition, 4 FL parameters for fluorescent characterization and subpopulation analysis.
