My Lab, along with the Saper group, is dedicated to advancing research in sleep and respiration, with a particular emphasis on the neural circuits involved in sleep, narcolepsy, sleep homeostasis, sleep deprivation, pain, and apnea-induced arousals. Our research employs state-of-the-art techniques such as optogenetics and pharmacogenetics to thoroughly investigate the neural circuits. Comprehensive recordings of sleep, respiration, and body temperature are conducted using mouse models. Furthermore, the lab utilizes sophisticated imaging methods, including fiber photometry for cell-population recordings and Inscopix-GRIN lens endoscopy for imaging individual cells. These methodologies facilitate a detailed analysis of specific neuronal activity and its correlation with respiration, sleep, and body temperature.
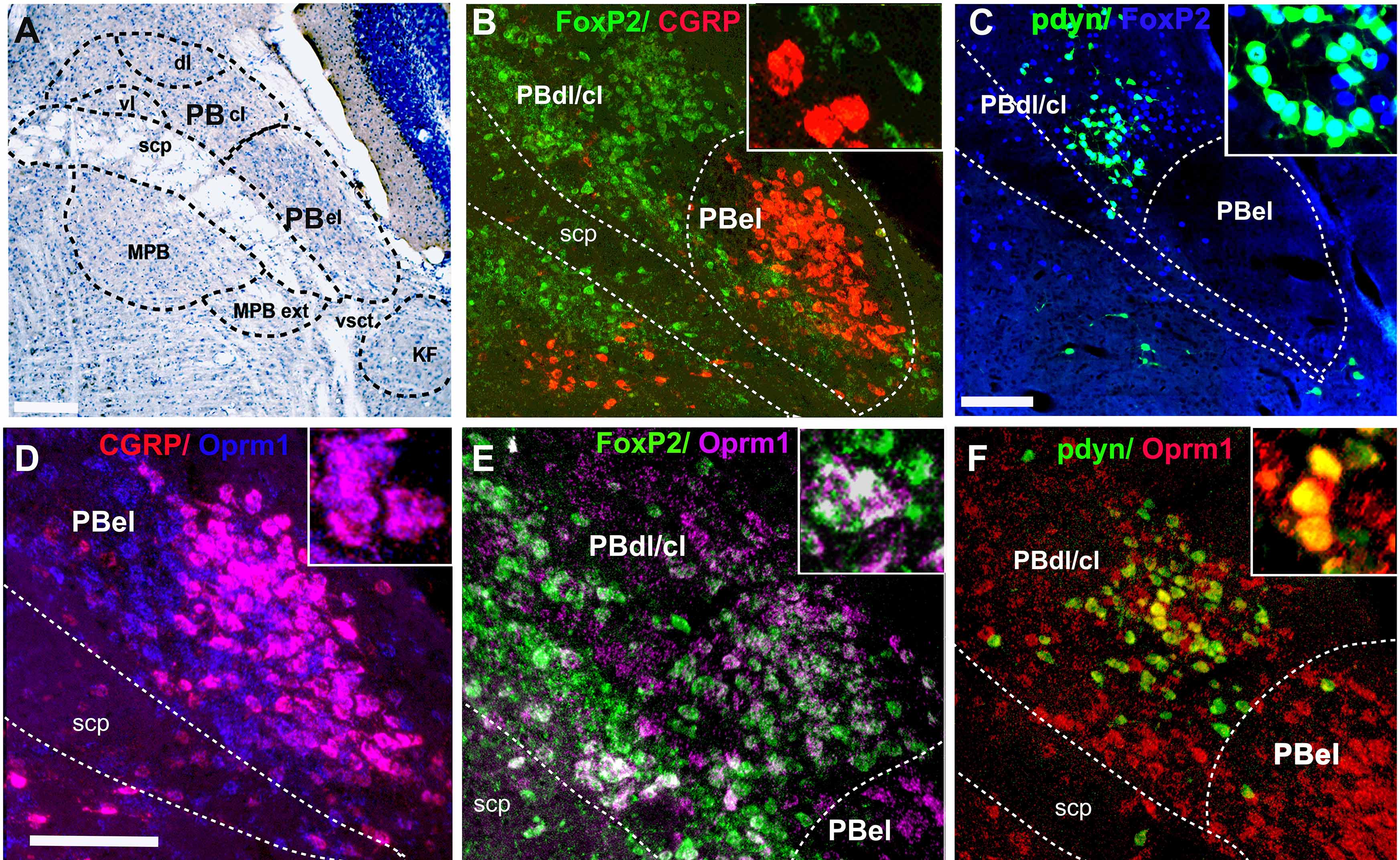
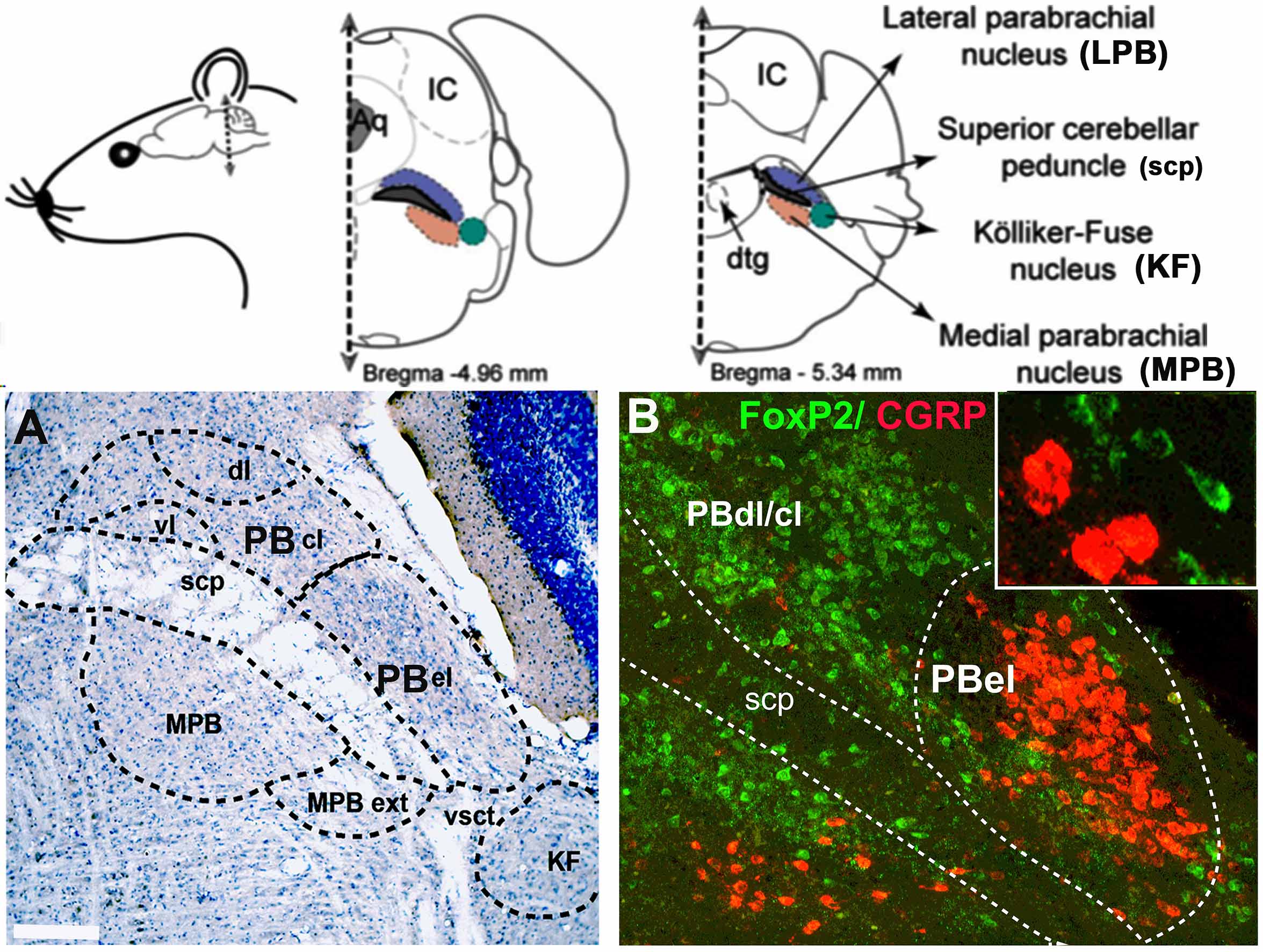
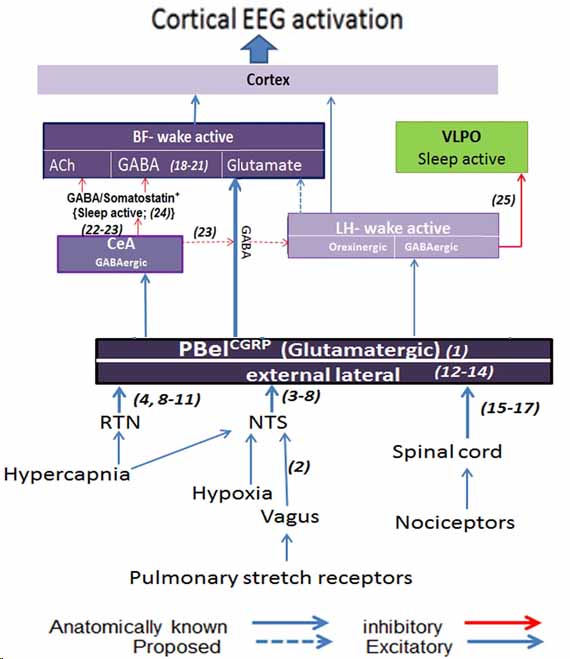
Our research has successfully characterized distinct cell types within the parabrachial (PB) area and their associated pathways that trigger cortical arousals in response to critical stimuli, including apnea and pain. Our findings elucidate the specific neuronal populations responsible for regulating cortical arousal and ventilatory responses during apnea. Importantly, these populations may also represent potential sites of action for opioids. Consequently, the research holds significant potential for developing innovative treatments for sleep disorders and enhanced strategies for pain management.
The mechanisms underlying waking up from apneas during sleep in patients with obstructive sleep apnea are not fully understood. Anatomical studies indicate that the parabrachial nucleus (PB) plays a crucial role by transmitting glutamatergic signals to key forebrain structures involved in arousal. Our research using a mouse model of apnea (repetitive hypercapnia stimulus), identified the involvement of specific PB neurons that express calcitonin gene-related peptide- CGRP (PBelCGRP) and serotonergic neurons in the dorsal raphe for promoting awakening (cortical arousals) during apneas through their actions on the 5HTreceptors (5Htr2A), while manipulations of these circuits did not affect the ventilatory responses
- Kaur S, Pedersen NP, Yokota S, Hur EE, Fuller PM, Lazarus M, Chamberlin NL, Saper CB. Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. J Neurosci. 2013 May 1;33(18):7627-40. PMCID: PMC3674488.
- Yokota S, Kaur S, VanderHorst VG, Saper CB, Chamberlin NL. Respiratory-related outputs of glutamatergic, hypercapnia-responsive parabrachial neurons in mice. J Comp Neurol. 2015 Apr 15;523(6):907-20. PMCID: PMC4329052.
- Kaur S, Wang JL, Ferrari L, Thankachan S, Kroeger D, Venner A, Lazarus M, Wellman A, Arrigoni E, Fuller PM, Saper CB. A Genetically Defined Circuit for Arousal from Sleep during Hypercapnia. Neuron. 2017 Dec 6; 96(5):1153-1167.e5. PMCID: PMC5720904.
- Kaur S, De Luca R, Khanday MA, Bandaru SS, Thomas RC, Broadhurst RY, Venner A, Todd WD, Fuller PM, Arrigoni E, Saper CB. Role of serotonergic dorsal raphe neurons in hypercapnia-induced arousals. Nat Commun. 2020 Jun 2;11(1):2769. PMC7265411.
The PB region is also the likely target for opioids to affect analgesia, sleep, and respiration (Lynch et al., 2023) by acting on different cell types. We recently showed the role of PBelCGRP neurons in causing sleep fragmentation in a chronic neuropathic pain model (Alexandre et al., 2024) using genetic deletions and manipulations. Additionally, we are testing their roles in acute inflammatory pain models (Lynch et al., 2024), which affect sleep and sleep fragmentation by acting on forebrain areas that mediate arousal. Our recent research also demonstrates that PBFoxP2 cells regulate respiration and the ventilatory response to CO2 (Kaur et al., 2024), making them a major site for opioids to cause respiratory depression.
- Lynch N, Lima JD, Spinieli RL, Kaur S. Opioids, sleep, analgesia and respiratory depression: Their convergence on Mu (μ)-opioid receptors in the parabrachial area. Front Neurosci. 2023; 17: 1134842. doi: 10.3389/fnins.2023.1134842. Review. PMC10117663.
- Alexandre C, Miracca G, Holanda VD, Sharma A, Kourbanova K, Ferreira A, Bicca MA, Zeng X, Nassar VA, Lee S, Kaur S, Sarma SV, Sacré P, Scammell TE, Woolf CJ, Latremoliere A. Nociceptor spontaneous activity is responsible for fragmenting non-rapid eye movement sleep in mouse models of neuropathic pain. Sci Transl Med. 2024 Apr 17;16(743): Epub 2024 Apr 17. PubMed PMID: 38630850.
- Kaur, S., Lynch, N., Sela, Y. et al. Lateral parabrachial FoxP2 neurons regulate respiratory responses to hypercapnia. Nat Commun 15, 4475 (2024) PMC11128025.
- Lynch N, Gangeddula N, Spinieli RL, Kaur S (2024) Brain circuits that regulate awakenings to pain stimulus. SLEEP 2024, (Volume 47 Supplement 1), A-82. 2024 June; Houston, TX, USA.