The Kim Laboratory investigates molecular and physiological mechanisms that regulate glucose and energy homeostasis, with a focus on pathways that are disrupted in obesity, type 2 diabetes and fatty liver disease. We concentrate on key signaling nodes, including the kinase ROCK1, the lipoprotein receptor LRP1, and hepatokines such as clusterin (ApoJ), that link liver, adipose tissue, skeletal muscle and the brain. Our approaches integrate mouse genetics, physiology and omics.
1. Central regulation & leptin transport (brain–body axis)
We study how brain barrier sites and hypothalamic circuits sense circulating hormones and coordinate energy balance.
Leptin entry mechanism: Work in choroid-plexus epithelial cells has identified a complex between short-form leptin receptors (LepR) and LRP1 that mediates transport of circulating leptin into the cerebrospinal fluid. Disruption of this complex reduces leptin entry into the brain, diminishes hypothalamic STAT3 signaling and promotes hyperphagia and weight gain, providing a mechanism for central leptin resistance in obesity.
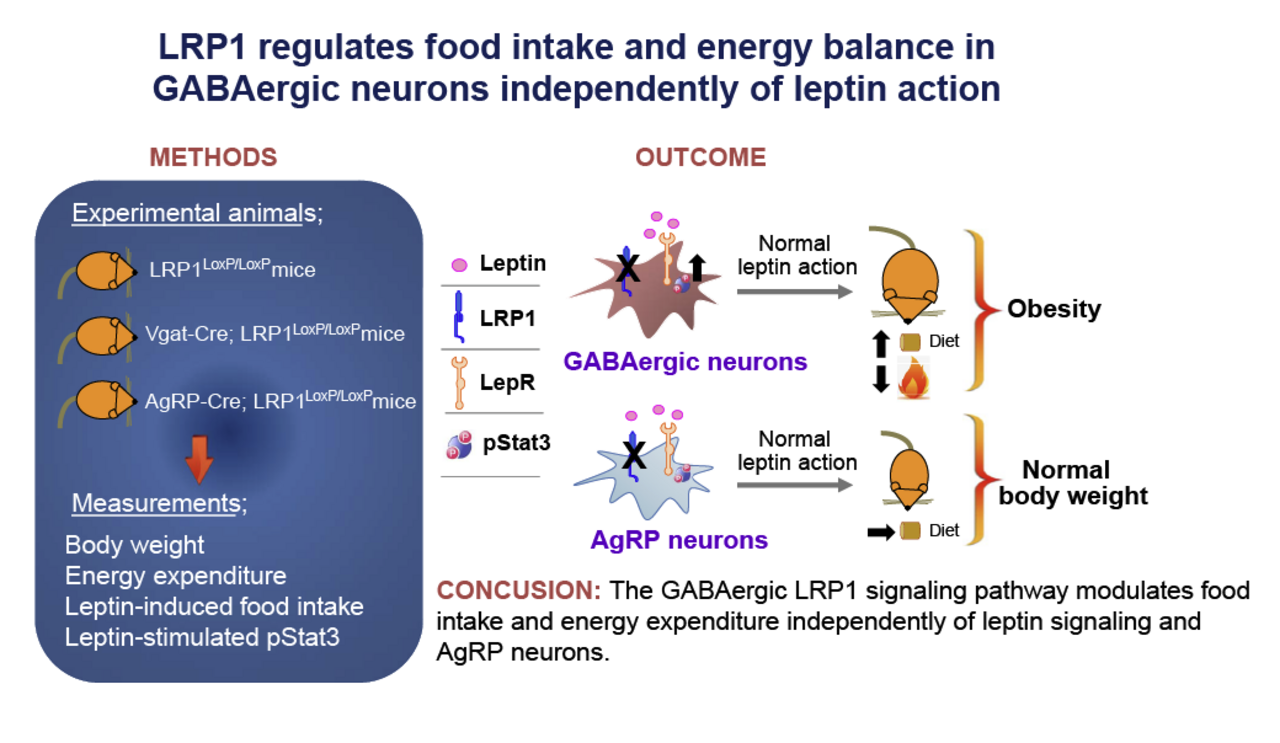
Hypothalamic signaling: Ongoing studies examine how hypothalamic Rho-kinase (ROCK1) and GABAergic circuits contribute to the regulation of food intake and energy expenditure.
2. ROCK1 in insulin resistance and fatty liver disease
We investigate ROCK1 as a stress-responsive kinase that links overnutrition to impaired insulin action and lipid accumulation.
Systemic insulin resistance: In liver, adipose tissue and pancreatic β-cells, ROCK1 activity interferes with insulin signaling and promotes de novo lipogenesis in the setting of high-fat feeding.
Therapeutic modulation: Genetic and pharmacologic studies in mouse models indicate that reducing ROCK1 activity improves insulin sensitivity and attenuates diet-induced fatty liver disease (NAFLD), supporting ROCK1 as a potential target in type 2 diabetes and related metabolic disorders.
3. Hepatokines and inter-organ crosstalk
We examine how liver-derived factors signal to peripheral tissues to shape whole-body metabolism.
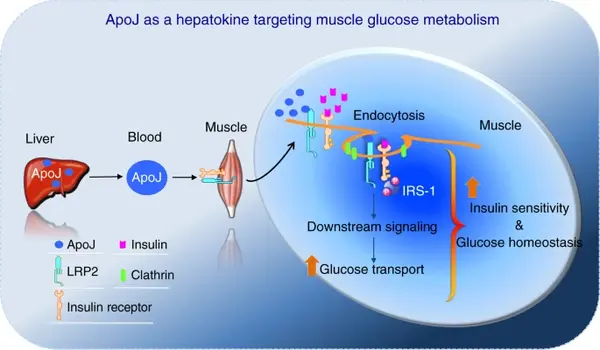
Liver–muscle axis: Work from the lab has identified clusterin (ApoJ) as a hepatokine that acts through the muscle receptor LRP2 to regulate insulin receptor internalization, insulin signaling and glucose uptake in skeletal muscle, linking altered ApoJ–LRP2 signaling to systemic insulin resistance.
Secretome analysis: Additional projects use genetic mouse models, secretome profiling and in vivo metabolic phenotyping to map liver–muscle and liver–brain communication pathways and to define inter-organ nodes that may be leveraged for metabolic therapy.