Alcoholic Hepatitis Clinical and Translational Network Late Phase Clinical Trials and Observational Studies
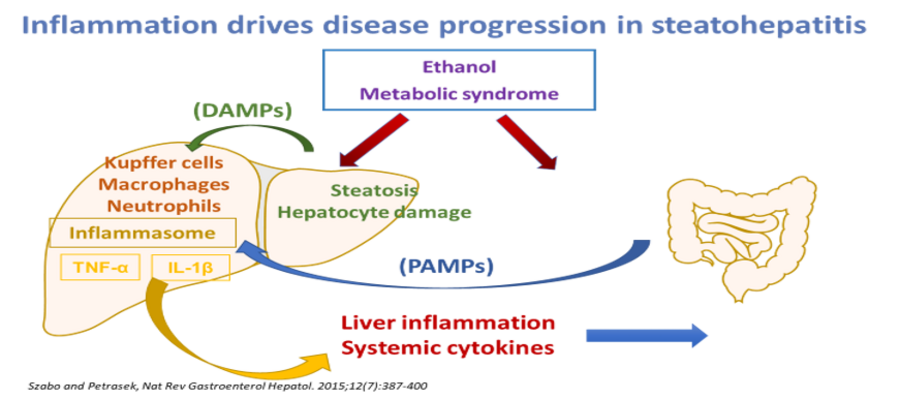
Alcoholic liver disease (ALD) is a major cause of liver-related morbidity and mortality in the US. Alcoholic hepatitis (AH) is a life-threatening form of ALD. Severe AH is associated with a mortality exceeding 30% at 3 months. Despite its obvious public health and economic burden, there is a remarkable paucity of effective therapeutics for severe AH. Corticosteroids have been the mainstay of therapy for AH, but are only modestly effective and increase the susceptibility to severe infections while pentoxifylline has recently been shown to be ineffective. There is an urgent need to develop safe and effective targeted therapies for severe AH. Anakinra, an IL1β receptor antagonist, zinc, and granulocyte colony stimulating factor (G-CSF) have been evaluated and have potential therapeutic benefit. Due to limitations in patient recruitment for clinical trials in general and AH in particular, there is a compelling need for an integrated national consortium for this deadly disease.
The use of the IL-1 receptor antagonist, anakinra, in this clinical trial is supported by preclinical studies in our group that demonstrated the role of NLRP3 inflammasome and the therapeutic benefit of anakinra in a mouse model of alcoholic liver disease.
Selected Publications:
