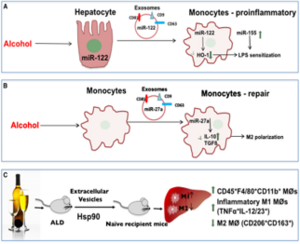
Macrophages (MØ), Kupffer cells (KC), and neutrophils mediate inflammation and play an important role in the pathogenesis of alcohol-associated liver disease (ALD). Previous studies have demonstrated the damaging effects of pro-inflammatory macrophages on alcoholic liver inflammation and high neutrophil infiltration in the liver predicted poor outcomes in human alcoholic hepatitis. Prior reports in humans and our preliminary data in mice show that both classically activated inflammatory and alternatively activated macrophages are present in the liver after chronic alcohol intake. However, the significance of macrophage (MØ) phenotypes or therapeutic targeting of MØ is yet to be explored in ALD. M2 “alternatively activated” macrophages have anti-inflammatory functions and contribute to tissue repair. M2 MØ phenotype is induced and modulated (regulated) by various factors including cytokines, microRNAs, transcription factors, or phagocytosis of apoptotic neutrophils. We found decreased phagocytic activity in alcohol-exposed MØ and observed decreased apoptosis in neutrophils isolated from livers of chronic alcohol-fed mice. Thus, we hypothesize that insufficient M2 polarization permits chronic inflammation and preferential M1 macrophage phenotype in the liver.