Research
Our laboratory has studied the optimal integration of molecular simulations into the generation of bioactive molecules. In collaboration with investigators at Harvard Medical School, we have applied this research methodology to discover novel immune modulators for the treatment of autoimmune diseases (e.g., Inflammatory Bowel Diseases, Diabetes, etc) and cancers (e.g., Breast cancer). Currently, our interest focuses on Aryl Hydrocarbon Receptor (AHR) modulators, Orai channel blockers, and threonine/serine kinases.
Aryl Hydrocarbon Receptor Modulators
The aryl hydrocarbon receptor (AHR) is an essential regulator of the gut's innate immune system and mediates processes, including the expression of interleukin-22 (IL-22), which is responsible for gut barrier function and microbial homeostasis. Our laboratory, in collaboration with Dr. Elliot Chaikof (BIDMC, Harvard Medical School), has been working on the discovery of AHR modulators. Utilizing a structure-based drug design approach, we have discovered PY108 and PY109 – two very potent AHR agonists with nM EC50 (Chen et al Sci Adv. 2020;6(3):eaay8230). In a DSS-induced colitis mouse model, treatment with PY109 or PY108 significantly improved survival, reduced weight loss, preserved colon length, and reduced overall disease activity. More importantly, these compounds also have promising physicochemical properties and DMPK profiles, which position them as excellent lead molecules for further development.

CRAC Channel Blockers
The Orai calcium channels control Ca2+ influx in lymphocytes and are crucial for store-operated Ca2+ entry (SOCE). A recessive loss-of-function mutation in Orai1 results in severe combined immunodeficiency (SCID) syndrome, supporting Orai as an attractive target for the development of selective immune modulators. We have recently discovered a class of indazole-3-carboxamides that potently and selectively block SOCE via the Orai channel. In HEK293 cells transfected with the constitutively active human Orai variant (G98S), patch-clamp electrophysiological measurement of calcium currents determined the IC50 of lead compounds as 3-4 μM. Two unique features distinguish our blockers from all known SOCE inhibitors - one is that they demonstrate a fast-onset mode of action and elicit complete blockade of Ca2+ influx without preincubation. The other is that washout experiments indicate that their inhibitory activity is reversible. Moreover, at concentrations as high as 100 μM, our lead compounds did not affect TRPM4 and TRPM7 channels, which are highly expressed in immune cells and are known to be indiscriminately affected by commonly used pharmacological tool compounds, including BTP2 (YM-58483). Consistent with their blockade of Orai channel, the lead compounds dose-dependently inhibited the nuclear translation of NFAT in Jurkat T cells and the proliferation of primary mouse immune cells.
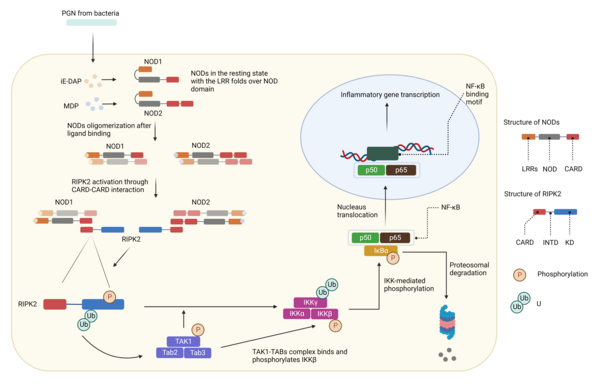
Threonine-Serine Kinase Inhibitors
Kinases play crucial roles in cellular signaling, including both innate and adaptive immune responses. In our laboratory, we are actively engaged in discovering small-molecule inhibitors targeting kinases involved in the pathology of autoimmune diseases and cancer. Our specific targets include RIPK2 and DYRK1A. Dysregulation of RIPK2 signaling has been implicated in the development of various inflammatory diseases, such as sarcoidosis, Blau syndrome, Crohn’s disease (CD), and ulcerative colitis (UC). Additionally, DYRK1A is upregulated in several types of cancers, including pancreatic ductal adenocarcinoma (PDAC) and non-small cell lung cancer (NSCLC).