NASA Projects
NASA Bedrest Study
This collaborative study, conducted in partnership with multiple research centers and the National Aeronautics and Space Administration (NASA), focuses on addressing the challenges of muscle atrophy and sensorimotor decline during space travel, simulated through the use of head-down bed rest. The goal is to evaluate various countermeasures that can preserve muscle health and function, which is critical for astronauts.
Our lab's role in this research is to evaluate the effectiveness of electrical muscle stimulation as a means to preserve muscle health and function. We will also employ advanced techniques, including electrical impedance myography and ultrasound imaging, to assess muscle condition and integrity. The findings from this study are expected to contribute to the development of strategies aimed at protecting astronaut health during and after spaceflight.
Lunar Living
With renewed interest in missions to the moon and the goal for astronauts to inhabit a lunar base in the future, it’s imperative that the physiological impacts of living on the moon be fully explored. On the lunar surface, astronauts will be exposed to unique environmental stressors including reduced gravity and galactic cosmic radiation. The “Lunar Living” study aims to investigate the acute and long-term effects of combined partial gravity and space radiation with the help of our friendly rat astronaut subjects.
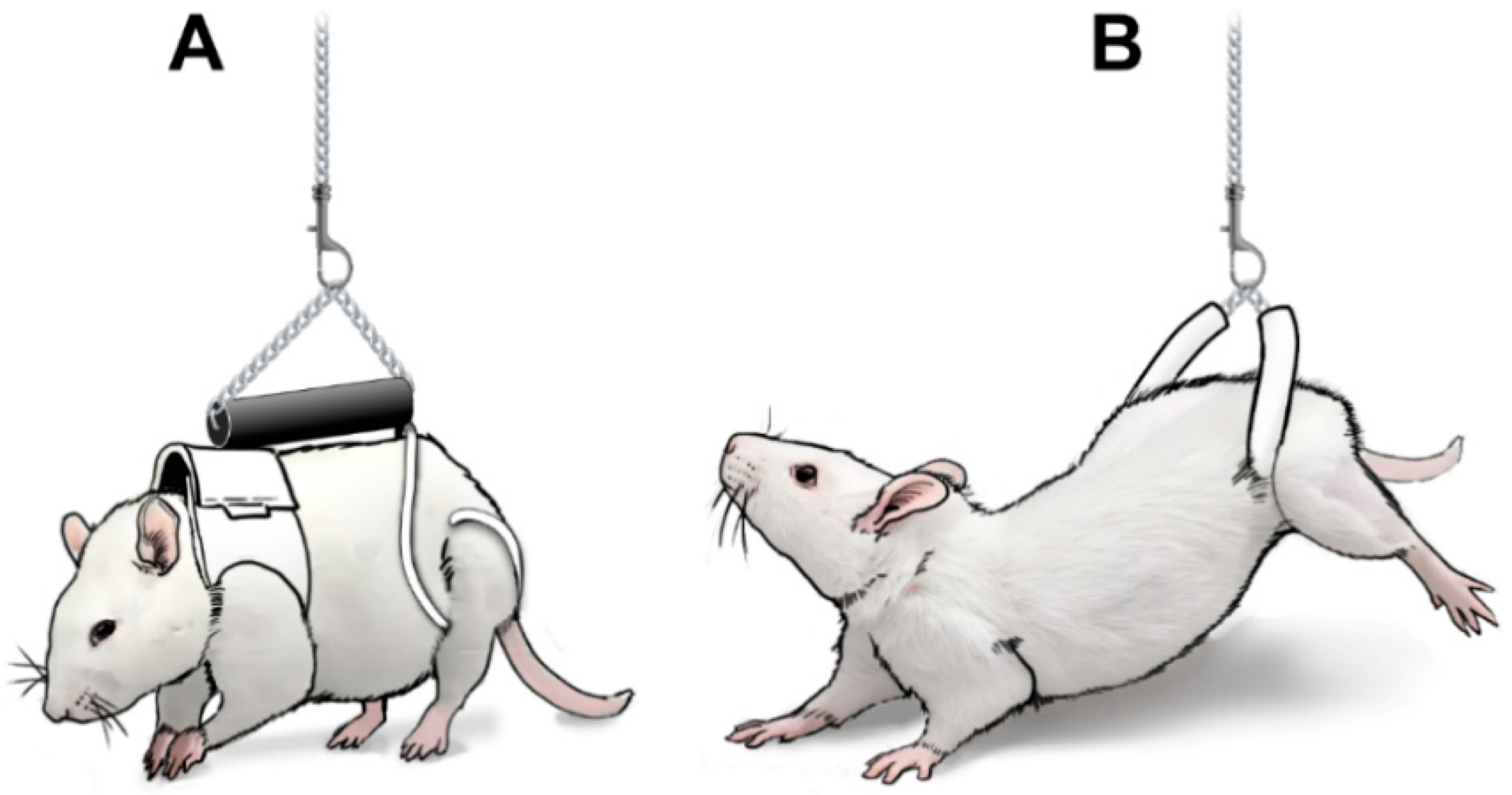
To simulate partial gravity, our team suspends rats in an apparatus such that the animal only supports ~20% of their total body weight. This analog model of partial weight bearing has been validated to simulate the muscle disuse experienced by astronauts on the moon. In collaboration with the Brookhaven National Laboratory (BNL) and NASA Space Radiation Laboratory, the animals also receive a one-time dose of radiation proportionate to the duration of their time in space. One cohort of animals is used to understand the acute effects of these stressors on musculoskeletal and neurological health/function, while a second cohort is returned to full weight bearing and is allowed to recover for 3 months at Beth Israel. During this time, recovery from “space flight” is monitored, allowing us to assess the long-term effects of space stressors.
We are excited to announce that the first Lunar Living campaign has been completed as of August 2024 and we are looking forward to the second campaign in Spring 2025.
Partial weight bearing model in rats:
Muscle and Metabolism in Alzheimer's Disease
Alzheimer’s disease (AD) is a debilitating form of dementia that affects approximately 6.7 million Americans over the age of 65. Despite the prevalence of AD, safe and efficacious therapies to treat AD are lacking. Although treatments targeting the amyloid-beta plaques associated with AD have been developed, these drugs are prohibitively costly and can result in serious complications including cerebral edema and hemorrhage. Thus, the development of alternative AD therapies is critical.
While AD is a well-characterized cognitive disorder, it is important to note that AD has effects in other tissues including skeletal muscle. Interestingly, neuronal tissue and skeletal muscle both accumulate amyloid-beta plaques prior to cognitive declines. Further, muscle wasting and weakness are also associated with AD and increased weakness is correlated with greater cognitive decline. Therefore, remodeling events that occur in both tissues may contribute to the development of AD. Of note is the metabolic dysfunction observed in both tissues prior to the onset of muscle weakness and cognitive deterioration. The important link between muscle and metabolism in the development of AD presents a potential alternative target for AD therapies. Because of its potential to modulate metabolism in the muscle and the brain, resistance exercise may be a viable alternative therapy to prevent AD. In fact, benefits from resistance exercise have been shown in animal models of AD and in humans with early-stage AD. However, the underlying mechanisms of these effects are poorly understood.
Thus, the aim of our work is to determine the impact of resistance exercise on metabolism and cognition in a pre-clinical model of AD. To address this, we will subject transgenic Alzheimer’s mice and wild type controls to 9 weeks of resistance exercise. Throughout this period, we will monitor muscle, metabolic, and cognitive function. After the training intervention, we will perform histological, biomolecular, and metabolomics analyses on muscle and neuronal tissue. The findings from this work will identify potential mechanisms of resistance exercise effects on brain health and function in AD. Further, the metabolomic profile will assist in the identification of druggable targets to harness metabolism as a means of AD prevention and therapy.
