Our lab integrates multiple complementary approaches, including:
- genetic discovery in human cohorts,
- genome editing in zebrafish and human cell models,
- mass spectrometry–based metabolite profiling, and
- high-throughput screening of small-molecule libraries.
We apply these strategies across several ongoing projects aimed at uncovering the molecular mechanisms that drive disease and translating these insights into targeted therapeutic interventions.
Development of zebrafish platforms for neurotherapeutic discovery
For many neurodegenerative diseases, current therapies are only modestly effective. Amyotrophic lateral sclerosis is a particularly devastating disease often affecting people in the prime of their lives. To advance the development of more effective drugs for ALS, we are engineering new electrophysiological tools in zebrafish and creating a novel high-throughput platform for drug screening. In combination with unique zebrafish models of ALS (G93A-SOD1, TDP43, and C9orf72), this platform aims to identify novel small molecules to treat ALS, in addition to other neurodegenerative diseases. (Read Manuscript)
Mechanisms Linking Chemical Exposure to Neurological Disease
The prototypic mitochondrial poison, cyanide,
is a potent neurotoxicant that induces Parkinsonian-like syndrome. Identifying the broad spectrum of metabolic derangements secondary to cyanide toxicity may highlight pathways for therapeutic intervention to prevent damage to the nervous system caused by chemical exposures. The overarching goal of these metabolic studies is to explore how metabolism can be redirected to mitigate the effects of environmental exposure to metabolic poisons. These discoveries pave the way for the development of therapies that are fundamentally different from existing therapeutics and have the potential to transform our ability to respond to neurotoxic chemicals. This project leverages the mass spectrometry facility in our department, which houses four LC-MS metabolomics platforms. (
Read Manuscript)
Drug discovery and preclinical studies that meet FDA definition of readiness for advanced drug development
We are part of a consortium seeking to develop a deployable cyanide antidote for mass casualty incidents. Our lab developed a series of high-throughput chemical screens. These scalable assays monitor a wide range of morphological, physiological, molecular, and behavioral phenotypes, including glucose homeostasis, mitochondrial potential, cardiotoxicity, neurotoxicity, and survival in zebrafish. To optimize the potency and minimize the toxicity of hit compounds, we perform structure-activity relationship studies to elucidate the physicochemical features, ligands, and formulations that influence their efficacy and safety. From these studies, a lead compound emerged, which exhibits efficacy in zebrafish, mice, rabbits, and pigs.
Currently, this compound is being optimized to meet the formal requirements for advanced development and clinical deployment. (Read Manuscript)
Illuminating the function of the understudied "druggable" genome using human phenotypes and model organisms
Despite the central role of kinases and GPCRs in human biology and disease, a substantial portion of the human kinome and gpcr-ome remains poorly characterized.
Defining the functions of these understudied genes holds the potential to uncover novel biological mechanisms and drive the development of transformative therapeutic strategies. Our group has assembled unique, large-scale human datasets that integrate natural genetic variation with proteomic and metabolomic profiling (~5,000 analytes), enabling us to map the genetic architecture of the understudied human genome. To functionally validate and dissect these genetic associations, we employ genome editing and high-throughput screening approaches in both zebrafish and human cell lines. This integrated platform allows us to systematically uncover the roles of previously uncharacterized druggable genes and accelerate the discovery of novel therapies.
(Read Manuscript)
Genetic and small molecule elucidation of metabolic diseases
By building discovery pipelines that utilize biochemical and ‘omics approaches, we interrogate the fundamental pathways of metabolic diseases.
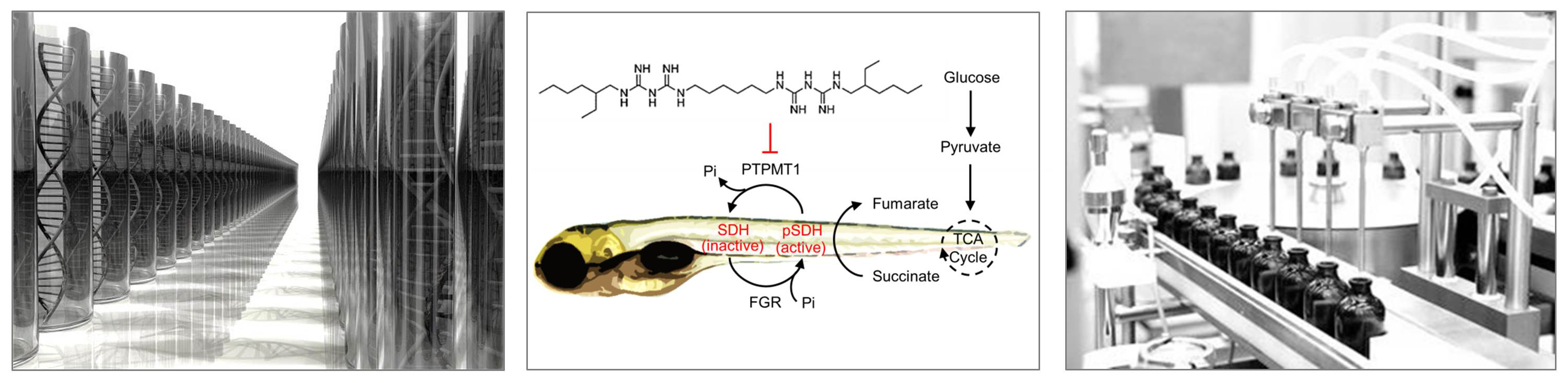
The overarching goal of these studies is to understand the natural history of the disease, thereby pinpointing candidate therapeutic targets. We then utilize the tools of chemistry and chemical biology in combination with genetic models in zebrafish to study these novel therapeutic entry points. We also utilize phenotypic-based screens for unbiased discovery, followed by target deconvolution. For example, we developed a high-throughput drug screening platform for energy homeostasis in zebrafish. Through this screen, we identified phosphorylated succinate dehydrogenase (SDH) as a novel substrate of the phosphatase PTPMT1. Establishing a link between PTPMT1 and SDH not only ascribed a new function to a poorly understood phosphatase (PTPMT1) but also revealed a novel molecular regulatory mechanism for SDH, an enzyme that is essential for mitochondrial function and the coordinated utilization of glucose.
(Read Manuscript)