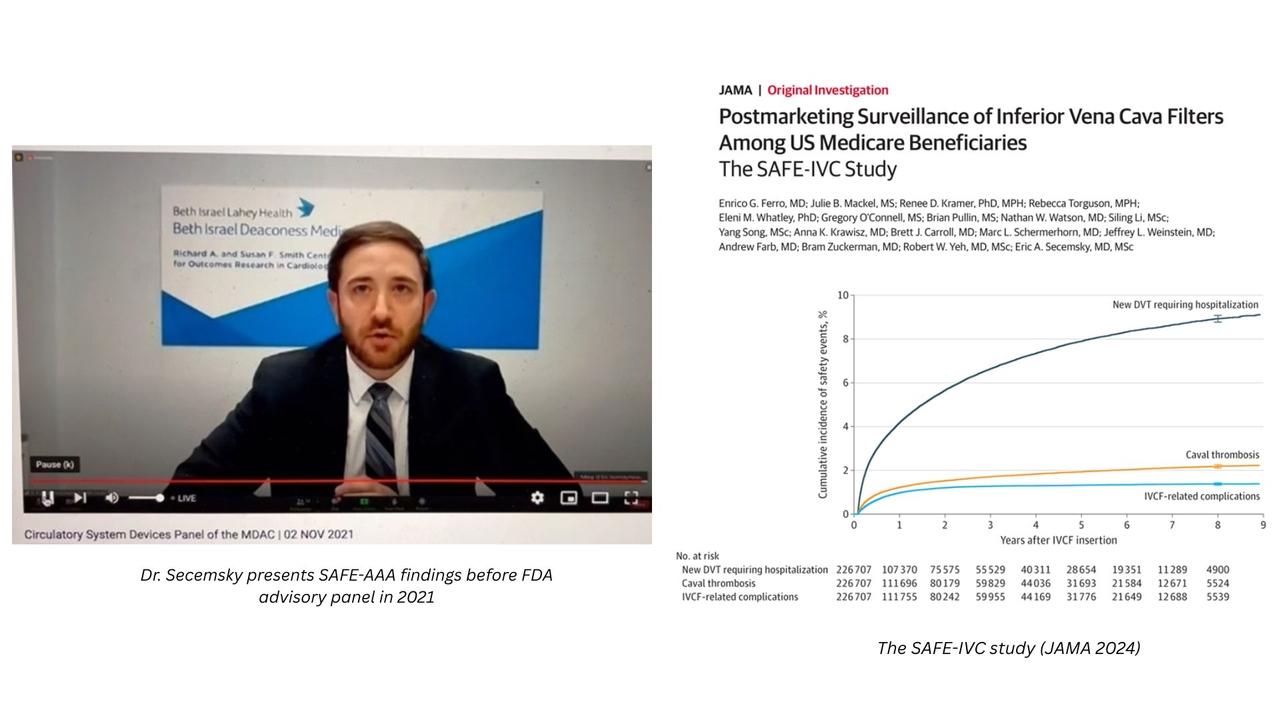
The work of the Interventional Cardiology and Vascular Research section has had important implications for cardiovascular device regulation.
Our section has been deeply involved in regulatory science, in particular the generation of timely safety data for post-market device evaluation. Our group has been contacted directly by the FDA to perform comparative research of FDA-approved medical devices, including drug-coated peripheral devices (SAFE-PAD), aortic aneurysm stent grafts (SAFE-AAA) and inferior vena cava filters (SAFE-IVC). To circumnavigate the limitations of sample size and generalizability often posed by randomized clinical trials and use of clinical registry data, these projects harnessed large, real-world observational datasets from nationwide insurance claims databases and applied state-of-the-science analytical methods to answer key device safety questions. Recognizing the significant scope and caliber of these studies, the FDA has invited Dr. Secemsky to present this work publicly at two national FDA Medical Device Advisory Committee meetings.
Notably, the SAFE-PAD study, which found no greater risk of mortality associated with paclitaxel-coated peripheral devices as compared to non-drug-coated devices in Medicare beneficiaries, was credited in part for the FDA's 2023 decision to reverse its 2018 advisory cautioning against use of paclitaxel-coated devices in femoropopliteal revascularization. In early 2025. the Centers for Medicare and Medicaid Services (CMS) cited SAFE-PAD as a model for real-world data (RWD) study protocols in their proposed guidance for the conduct of RWD studies.