Research
Liver Organoids
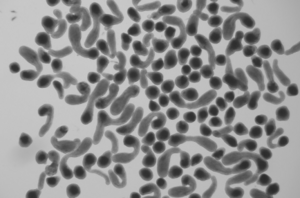
Liver organoids are three-dimensional, miniaturized liver-like structures grown from stem cells or primary liver cells in vitro. They mimic key aspects of liver function, including metabolism, detoxification, and albumin production. These organoids are used for disease modeling, drug testing, regenerative medicine, and studying liver development. In tissue engineering, liver organoids play a crucial role in developing bioengineered liver tissues for transplantation, offering a potential solution for liver disease treatment and organ shortages. Their ability to self-organize and replicate liver tissue architecture makes them a promising tool for biomedical research and regenerative medicine.
Pancreatic Organoids
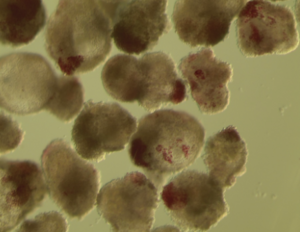
Pancreatic organoids are three-dimensional, miniaturized pancreatic tissue models grown from stem cells or primary pancreatic cells in vitro. They replicate key functions of the pancreas, including enzyme secretion and hormone production, making them valuable for studying pancreatic development, disease modeling (such as diabetes and pancreatic cancer), and drug testing. In tissue engineering, pancreatic organoids hold potential for regenerative therapies, offering new avenues for treating pancreatic disorders and developing bioengineered pancreatic tissues for transplantation.
Intestinal Organoids
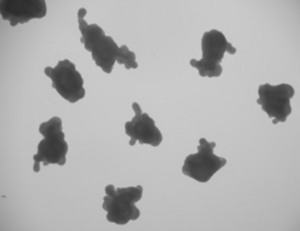
Intestinal organoids are miniature, lab-grown models of the intestine that closely resemble its structure and function. Developed from stem cells, they contain key cell types found in the gut and can perform essential processes like absorbing nutrients and maintaining a protective barrier. These organoids serve as powerful tools for studying diseases, testing drugs, and exploring interactions with the microbiome. In the field of tissue engineering, they offer promising possibilities for regenerating damaged intestinal tissue and developing new treatments for digestive disorders.
Recent Publications
SCORE Center
Beth Israel Deaconess Medical Center
Research North, Room RN0245
99 Brookline Avenue
Boston MA, 02215
