TSP-1 is a critical anti-angiogenic protein that counterbalances the pro-angiogenic activity of various factors, including VEGF, in normal physiology. Solid tumors are characterized by a tortuous vasculature, reduced perfusion, hypoxia, and high interstitial fluid pressure which impairs entry of therapeutic compounds into the tumor. Fc3TSR, like other anti-angiogenic reagents, specifically targets dysfunctional vasculature that lacks pericyte coverage, pruning the tumor vasculature back to more normal, mature vessels, a process termed vasculature normalization. This results in a tumor that has enhanced perfusion, reduced hypoxia and reduced interstitial fluid pressure.
We have determined the effect of Fc3TSR on two cancers that are refractory to treatment, epithelial ovarian cancer (EOC) and pancreatic cancer in collaboration with the laboratories of Jim Petrik and Dipak Panigrahy. EOC is the most lethal cancer of the female reproductive organs, with most women being diagnosed with metastatic spread within the peritoneal cavity. Fc3TSR is specifically potent in the treatment of EOC in murine models because it concurrently targets multiple key pathways in progression (Fig. 1). Fc3TSR induces apoptosis of both EOC and endothelial cells through the membrane protein CD36, which is expressed on both cell types. Fc3TSR also reduces the expression of cytokines that are immunosuppressive in the context of ovarian cancer, including interleukin 6 (IL-6), transforming growth factor beta (TGFbeta), and vascular endothelial growth factor (VEGF), by EOC cells. Fc3TSR significantly increases tumor perfusion, improves the uptake of chemotherapeutics by tumors and promotes immune cell trafficking. Consistent with these results, combining Fc3TSR with first line chemotherapeutics produces the greatest increase in survival.
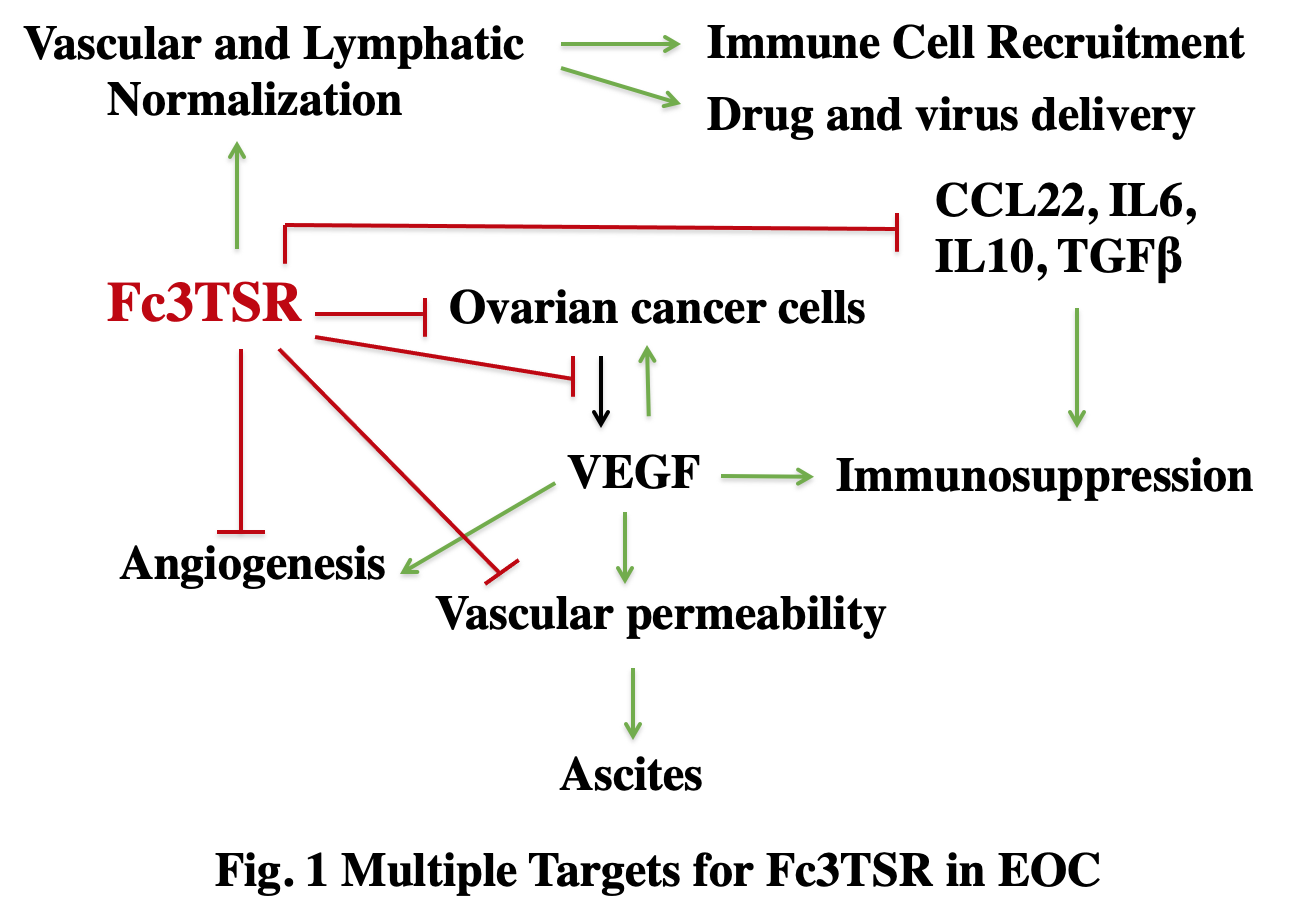
EOC and pancreatic cancer are similar in that (1) they are usually detected at a late stage when the disease has spread, (2) the tumors are poorly perfused, (3) the tumors are immunosuppressive and resistant to immunotherapy, and (4) ascites accumulation is a significant component of morbidity. Approximately 20,000 women and 50,000 patients die each year in the U.S. from ovarian and pancreatic cancer, respectively. The high mortality relates to the facts that (1) most patients are diagnosed with metastatic disease and (2) there is a lack of effective second-line therapies for patients who relapse. Unfortunately, the five-year survival rate for ovarian and pancreatic cancer has changed very little over the last three decades and new treatment options are urgently needed.
We have found the Fc3TSR is more active than the monomeric form of the anti-angiogenic domain of TSP-1 both in vitro and in vivo. The increased activity may stem from increased exposure and the fact that Fc3TSR contains two copies of the active domain, which facilitates clustering of CD36 and enhances its ability to signal through the src family kinase, Fyn. Importantly, we have published that the unfused anti-angiogenic domain of TSP-1, designated 3TSR, is more effective than the current first-line chemotherapy regimen in a murine model of EOC and further reduction in tumor growth is achieved by combining 3TSR and chemotherapy (Russel et al. 2015).
Fc3TSR is an Fc fusion protein that contains two copies of the anti-angiogenic domain of human TSP-1 at the C-terminus of the Fc fragment of human immunoglobulin G, fused via a flexible (Gly4Ser)3 linker. Our current version of Fc3TSR carries the LALA and P329G mutations so as to eliminate the immune effector functions mediated by FcgR and complement binding. This should minimize potential immune side effects in both human and mouse. We have demonstrated that Fc3TSR is drug-like in that it can be readily produced with high yield and purity using standard methods for protein production, is active in vivo at low concentrations (0.05 to 0.25 mg/kg/week), is stable, and has a half-life in mice that is approximately five days (Matuszewska et al. 2021). Our data indicate that Fc3TSR could likely be delivered through intravenous or intraperitoneal injection in the outpatient setting.
