TSP-1 is released from human blood platelets in response to thrombin treatment and was thus, designated thrombospondin in 1978. Since that time, four other members of the TSP gene family have been discovered. The TSPs are widely expressed in embryonic tissue. However, expression in adult tissue is much more limited and frequently involves sites of tissue remodeling or repair. Since TSP-1 was the first family member to be identified, and because it is readily purified from platelets, it is the most extensively studied. TSP-1 reportedly has important roles in angiogenesis, atherosclerosis, cancer progression and metastasis, ER stress response, immunity, ovarian follicle development, synapse formation, and wound healing. See the Resources Page for the links to recent review articles.
Whereas the TSPs share many properties with extracellular matrix proteins, they differ in that they do not self-associate to form fibrils. Rather, TSPs appear to remain associated with the cell surface where they regulate cell adhesion and migration, cell-to-cell interactions, and cellular phenotype. Proteoglycans, integrins, CD36, CD47, sulfatides and calreticulin function to sequester TSP-1 at the cell surface. Through these receptors, TSP-1 supports adhesion and migration of various cell types, including mammary tumor cells. When TSP-1-null mice are crossed with a genetically engineered model of breast cancer, the primary tumors grow more quickly as result of a more permissive environment for angiogenesis. Despite an increase in tumor burden, the TSP-1-null mice have significantly less lung metastasis. In addition, TSP-1-null tumor cells displayed decreased migration on collagen.
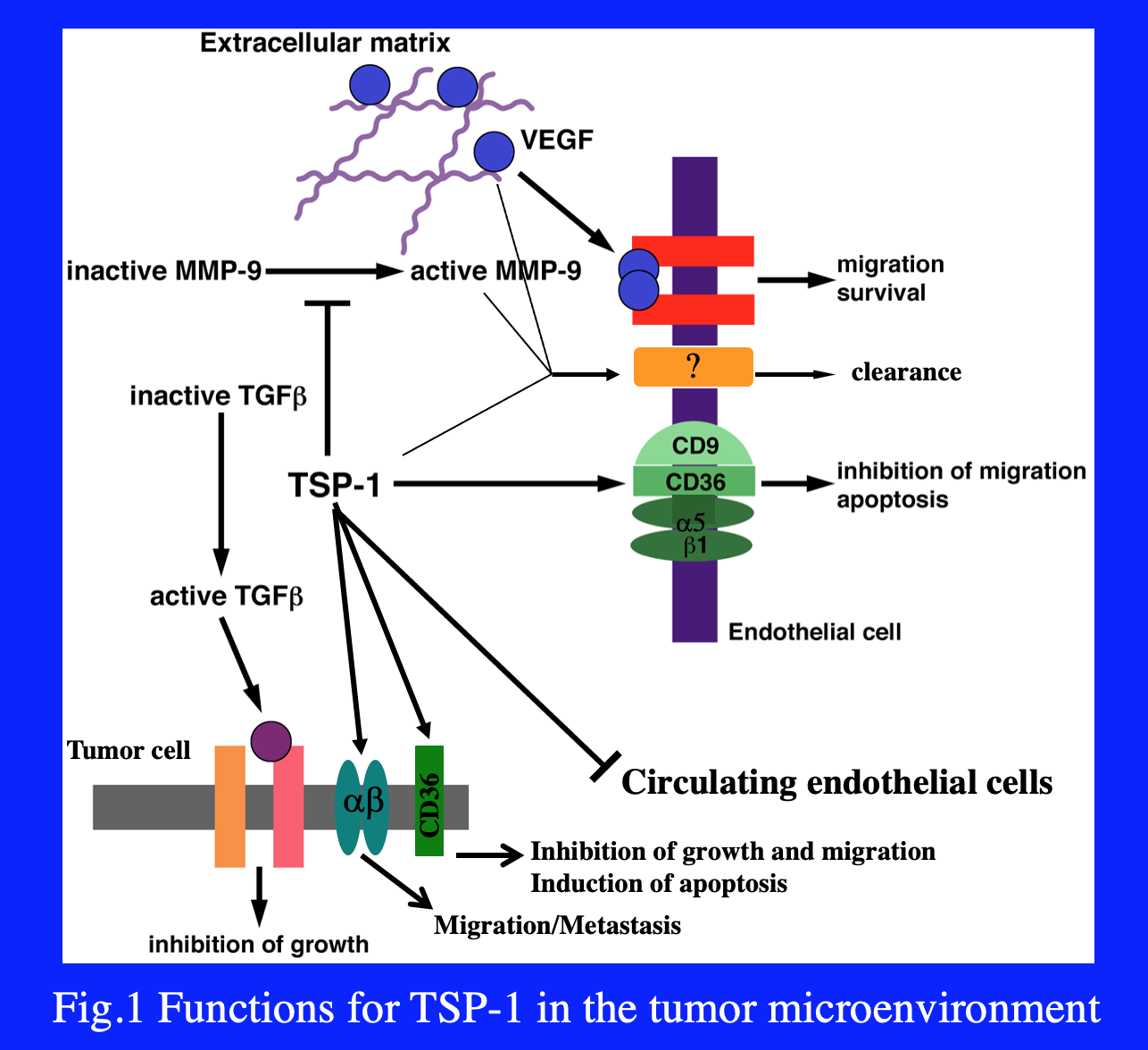
The Lawler lab has focused on the role of TSP-1 in the tumor microenvironment, where it has direct effects on angiogenesis, tumor growth and metastasis (Fig. 1). Many of the activities of TSP-1 in tumors antagonize VEGF availability or activity. TSP-1 inhibits the activation of MMP9, an enzyme that promotes mobilization of VEGF stored in the extracellular matrix. TSP-1 also binds to and promotes the clearance of VEGF. In the presence of VEGF, VEGFR2 is phosphorylated on multiple tyrosine residues, which in turn leads to activation of pro-survival and pro-migratory signal transduction pathways. In the presence of TSP-1, molecular complexes containing VEGFR2, CD36, SHP-1, integrins and tetraspanins form. The recruitment of the phosphatase SHP-1 to the complex by CD36 results in the dephosphorylation of VEGFR2 and the inhibition of the VEGF-induced signal transduction (Fig. 2). Taken together, the data imply that (1) TSP-1 has evolved as an anti-angiogenic molecule specifically designed to antagonize VEGF and (2) cross-talk between pro- and anti-angiogenic signal transduction pathways occurs at the endothelial cell membrane.
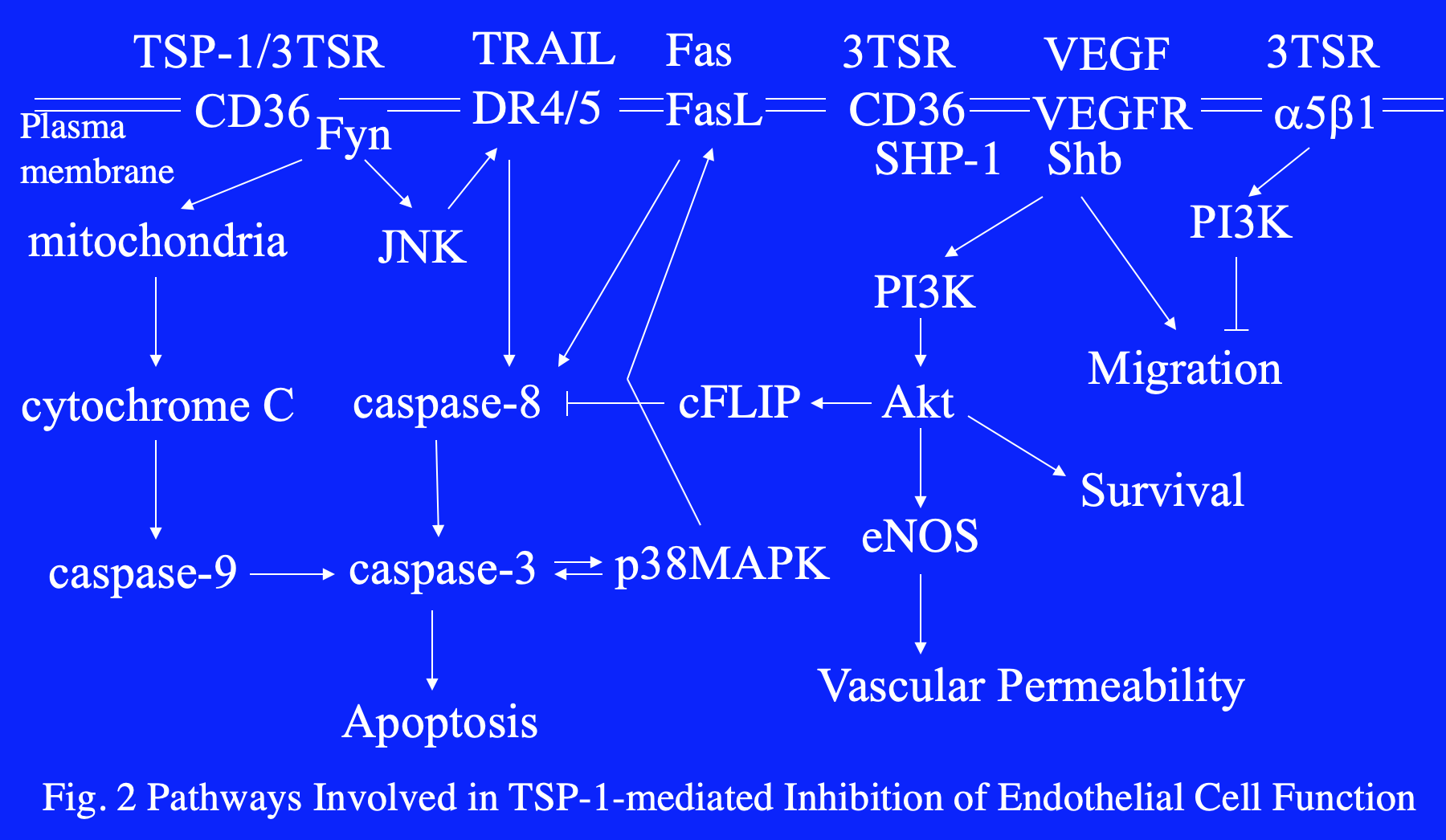
TSP-1 activates apoptotic pathways through CD36 in endothelial cells and in tumor cells that express it, such as those in ovarian and pancreatic cancer. TSP-1 also inhibits tumor growth through activation of TGFβ when the tumor cells, such as B16F10 melanoma, retain responsiveness to TGFβ. Interestingly, the activation of TGFβ by TSP-1 confers resistance to targeted drug therapy in thyroid cancer.
