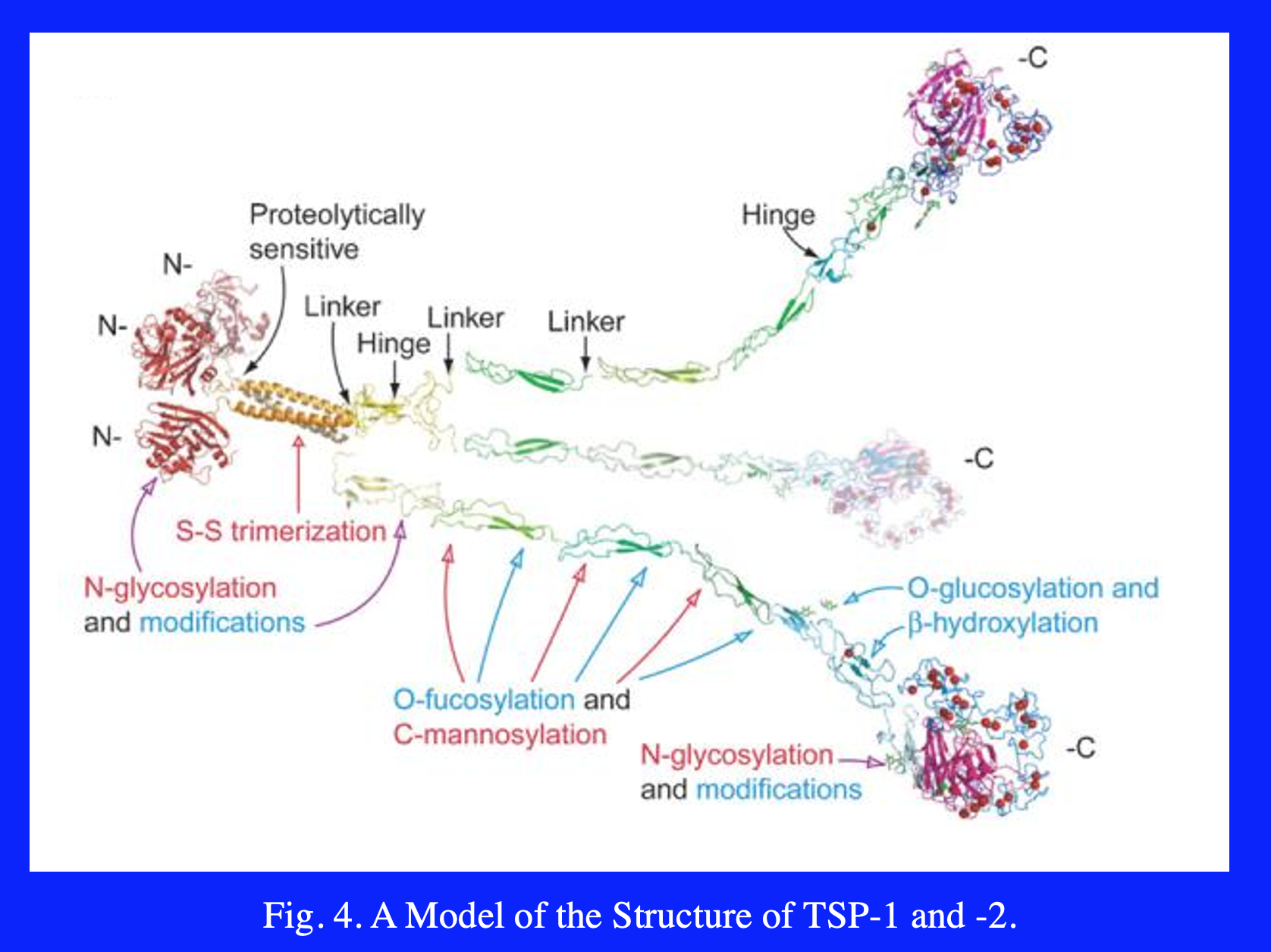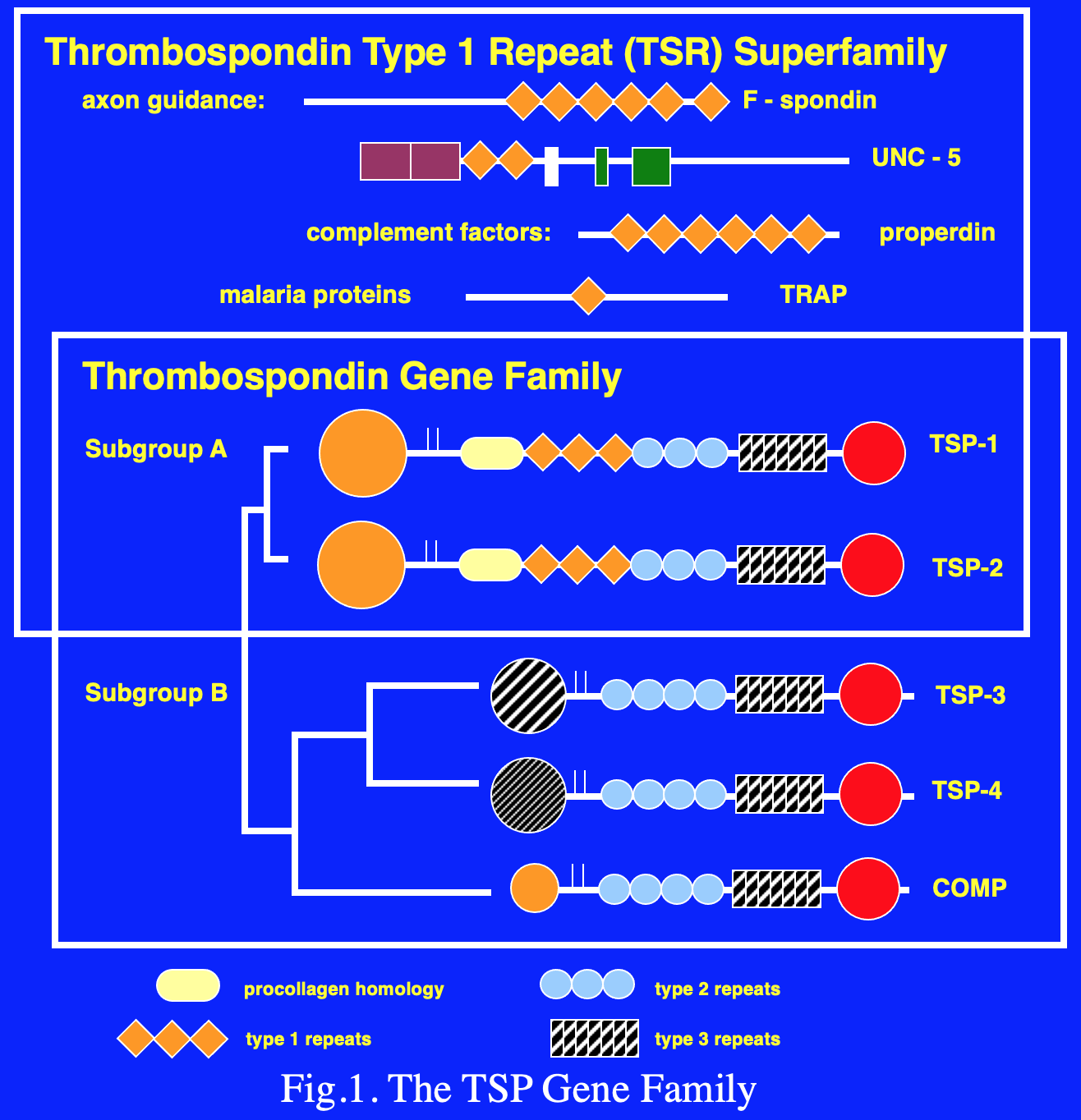
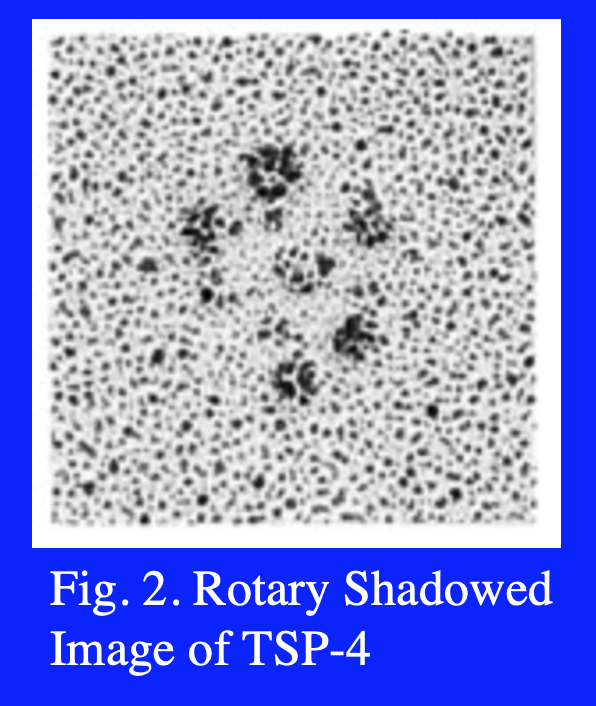
Five members of the TSP gene family have been identified (Fig. 1). TSP-1 and -2 (Subgroup A) are similar in their domain structure and the fact that they form disulfide-linked trimers. By contrast, TSP-3 and -4, and cartilage oligomeric matrix protein (COMP) (Subgroup B) assemble into pentamers. An electron microscopic image of rotary shadowed TSP-4 is shown in Fig. 2. The N-terminal domains frequently appear as a single large area of electron density (center of the molecule shown in Fig. 2) because the coiled-coil domains that drive multimer assembly are close by. The type 1 and 2 repeats appear as extended, solvent accessible regions, and the type 3 repeats and C-terminal domain appear as a single spherical region at the end of each strand of the pentamer.
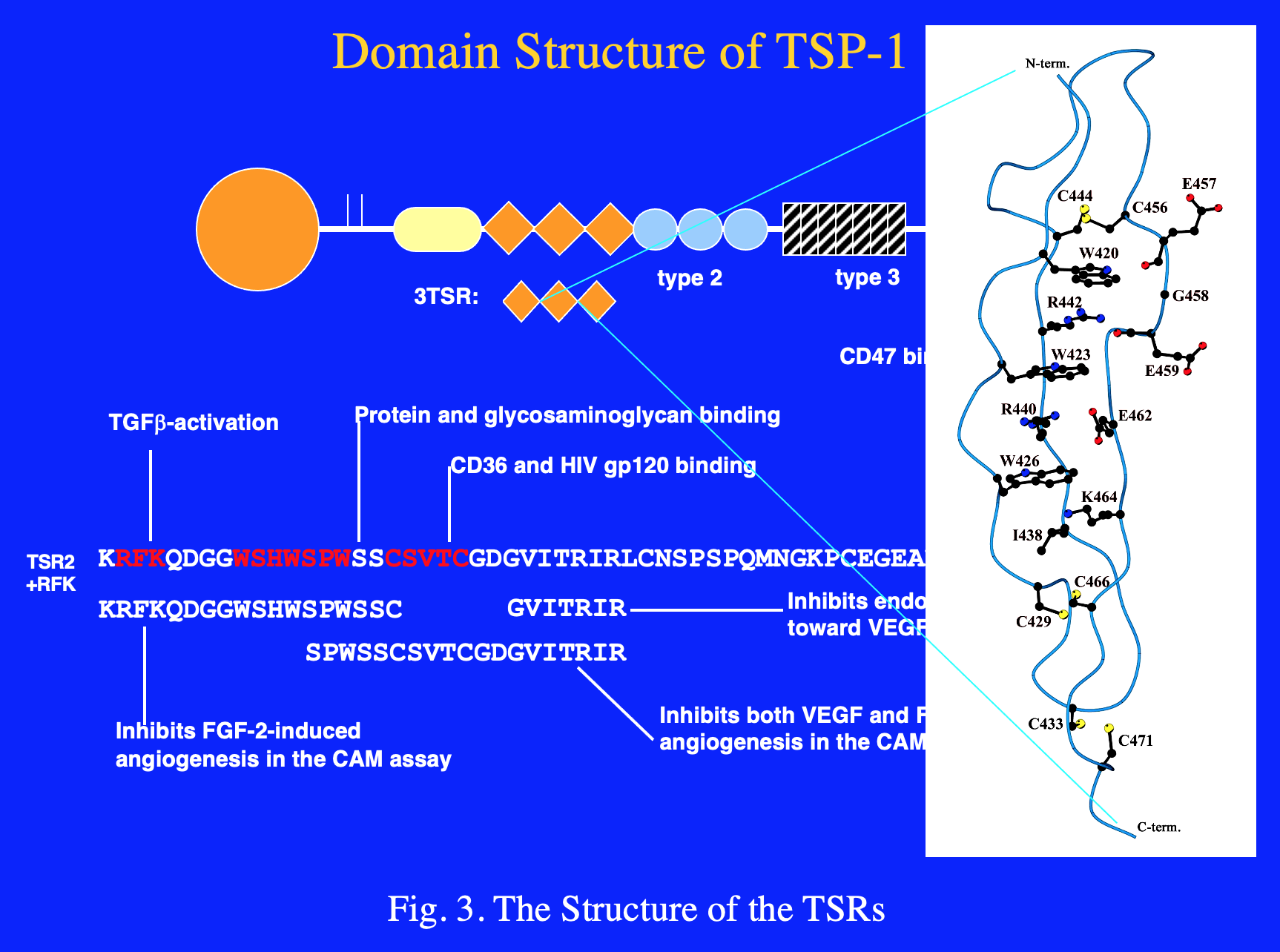
The amino acid sequences of TSPs revealed the presence of internal repeated structural domains that correspond to specific functional domains. The type 2 repeats are similar to the EGF repeats that are commonly found in extracellular matrix models and elsewhere. The subgroup B TSPs differ from those in subgroup A in that they have an additional type 2 repeat and lack the type 1 repeats (TSRs). The TSRs are characterized by a WSXWSXW sequence and an RIR sequence that together are key to forming a novel protein fold (Fig. 3). The TSRs are stabilized by cation-π bonds between conserved tryptophan (W) and arginine (R) residues, and by three intrachain disulfide bonds. It is likely that the conformation of the domain is essential for optimal CD36 binding and anti-angiogenic activity.
The highest level of sequence conservation is seen in the C-terminal half of the proteins and thus, this region is referred to as the “signature domain.” The type 3 repeats and the C-terminal domain comprise the majority of the signature domain. The type 3 repeats, constitute a contiguous series of calcium binding sites that are essential for correct folding and secretion of the protein. An RGD, integrin-binding motif, is present in the type 3 repeats. The αvβ3 integrin mediates RGD-dependent adherence of endothelial cells to TSP-1 in a calcium-dependent fashion. The type 3 repeats of each subunit have 30 calcium binding sites, and calcium also binds to the type 2 repeats and the C-terminal domain. This means that the pentameric TSPs can bind about 150 calcium ions. The physiological reason for the sequestration of all of this calcium is unknown. An adequate supply of calcium in the ER is essential for correct folding of the TSPs. Thus, the TSPs may serve to couple ER calcium levels with the unfolded protein response. The TSPs also bind stromal interaction molecule 1 (STIM1) and regulate the activity of calcium channels that reside in the plasma membrane. Based on conserved sequences in the canonical TSPs, Drs. Richard Tucker and Jo Adams have performed extensive analysis of a wide range of genomes to identify the members of a thrombospondin superfamily.
Domains from various members of the TSPs have been crystalized. Links to the crystal structures of the domains of various TSPs can be found on the Resources page. These include structures of the N-terminal domain of TSP-1 in the absence and presence of various forms of heparin and a glycosylated form of the TSRs. The crystal structures along with the electron microscopic images enable the development of a molecular model for the subgroup A TSPs (Fig. 4) (Carlson et al. 2008).