IMPORTANCE: The gold standard for outcome adjudication in clinical trials is medical record review by a physician clinical events committee (CEC), which requires substantial time and expertise. Automated adjudication of medical records by natural language processing (NLP) may offer a more resource-efficient alternative but this approach has not been validated in a multicenter setting.
OBJECTIVE: To externally validate the Community Care Cohort Project (C3PO) NLP model for heart failure (HF) hospitalization adjudication, which was previously developed and tested within one health care system, compared to gold-standard CEC adjudication in a multicenter clinical trial.
DESIGN, SETTING, AND PARTICIPANTS: This was a retrospective analysis of the Influenza Vaccine to Effectively Stop Cardio Thoracic Events and Decompensated Heart Failure (INVESTED) trial, which compared 2 influenza vaccines in 5260 participants with cardiovascular disease at 157 sites in the US and Canada between September 2016 and January 2019. Analysis was performed from November 2022 to October 2023.
EXPOSURES: Individual sites submitted medical records for each hospitalization. The central INVESTED CEC and the C3PO NLP model independently adjudicated whether the cause of hospitalization was HF using the prepared hospitalization dossier. The C3PO NLP model was fine-tuned (C3PO + INVESTED) and a de novo NLP model was trained using half the INVESTED hospitalizations.
MAIN OUTCOMES AND MEASURES: Concordance between the C3PO NLP model HF adjudication and the gold-standard INVESTED CEC adjudication was measured by raw agreement, κ, sensitivity, and specificity. The fine-tuned and de novo INVESTED NLP models were evaluated in an internal validation cohort not used for training.
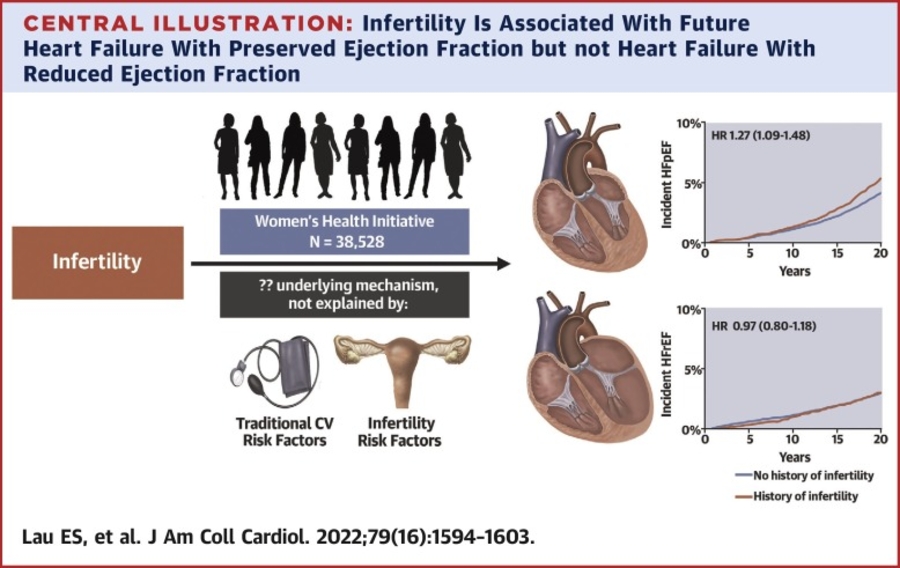
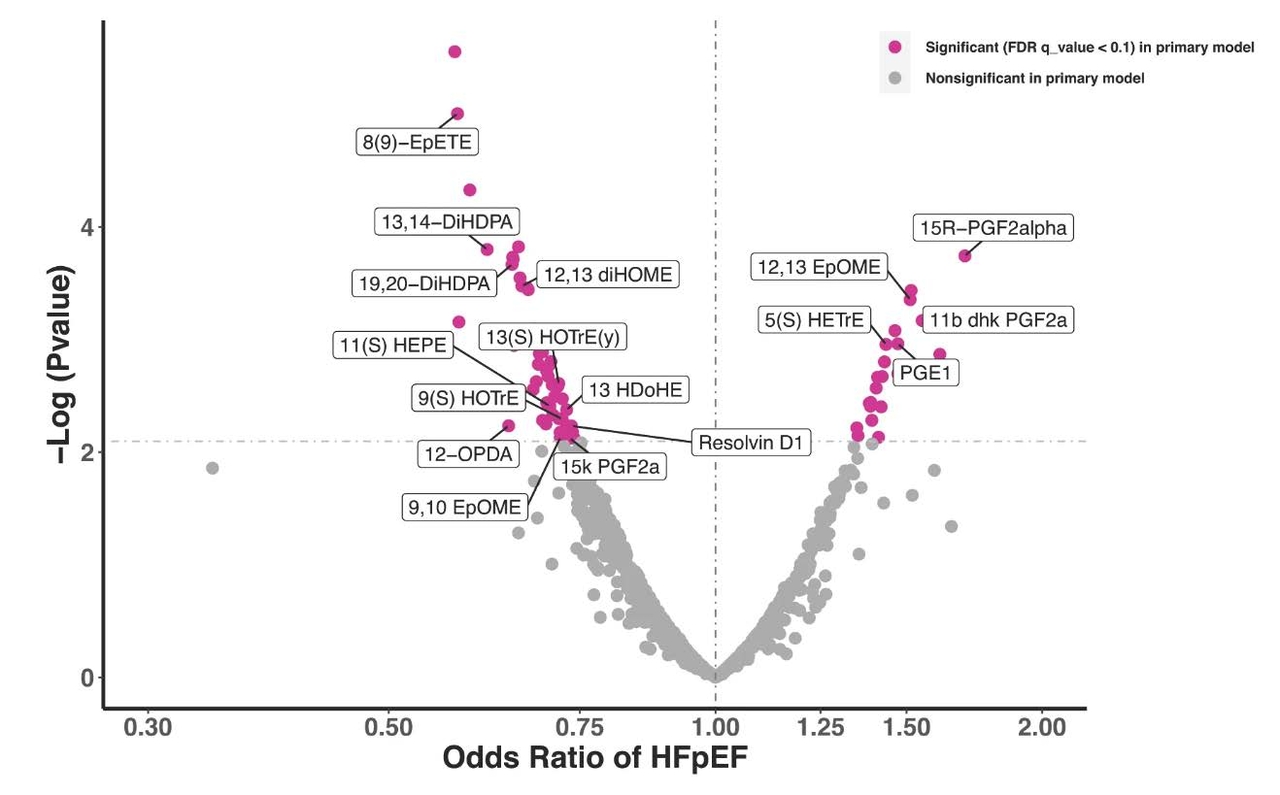
RESULTS: Among 4060 hospitalizations in 1973 patients (mean [SD] age, 66.4 [13.2] years; 514 [27.4%] female and 1432 [72.6%] male]), 1074 hospitalizations (26%) were adjudicated as HF by the CEC. There was good agreement between the C3PO NLP and CEC HF adjudications (raw agreement, 87% [95% CI, 86-88]; κ, 0.69 [95% CI, 0.66-0.72]). C3PO NLP model sensitivity was 94% (95% CI, 92-95) and specificity was 84% (95% CI, 83-85). The fine-tuned C3PO and de novo NLP models demonstrated agreement of 93% (95% CI, 92-94) and κ of 0.82 (95% CI, 0.77-0.86) and 0.83 (95% CI, 0.79-0.87), respectively, vs the CEC. CEC reviewer interrater reproducibility was 94% (95% CI, 93-95; κ, 0.85 [95% CI, 0.80-0.89]).
CONCLUSIONS AND RELEVANCE: The C3PO NLP model developed within 1 health care system identified HF events with good agreement relative to the gold-standard CEC in an external multicenter clinical trial. Fine-tuning the model improved agreement and approximated human reproducibility. Further study is needed to determine whether NLP will improve the efficiency of future multicenter clinical trials by identifying clinical events at scale.