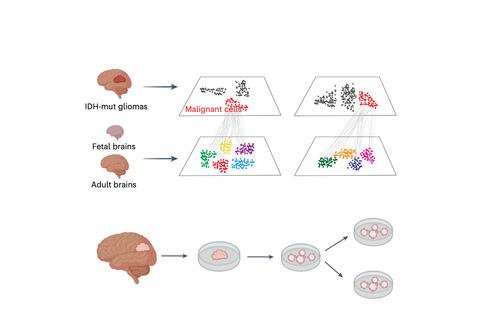
A collaborative team led by Dr. Jingyi Wu at the Dana-Farber Cancer Institute and Massachusetts General Hospital has revealed how epigenetic and genetic forces drive the deadly progression of IDH-mutant gliomas, brain tumors that initially grow slowly but almost always become fatal.
Published in Nature Cancer, the study combines cutting-edge single-cell genomics and organoid modeling to map, in unprecedented detail, how tumor cells evolve as the disease advances. The researchers found that early-stage IDH-mutant gliomas are powered by slow-dividing oligodendrocyte progenitor cell (OPC)-like cells, whose growth is sustained by DNA hypermethylation, an epigenetic signature that silences immune pathways and tumor suppressor genes.
Over time, these OPC-like cells partially reprogram into neural progenitor cell (NPC)-like cells, which are more proliferative and aggressive. As these cells divide, they lose DNA methylation and become dependent on genetic mutations, such as PDGFRA and MYCN amplifications and CDKN2A deletions, that further accelerate tumor growth and suppress the immune system’s interferon (IFN) defense.
“Our data reveal a striking switch from epigenetic to genetic control as IDH-mutant gliomas progress,” said Dr. Wu, first author of the study. “This helps explain why these tumors are initially slow-growing yet eventually become unstoppable.”
The team also showed that inhibiting DNA methylation either with a DNMT1 inhibitor or an IDH enzyme inhibitor can reactivate interferon signaling and promote differentiation of malignant cells toward a less aggressive state. These findings highlight a potential therapeutic window in early-stage tumors, when epigenetic therapies may still be effective before genetic alterations take over.
It provides a detailed molecular roadmap for glioma evolution and a foundation for developing treatments that target the disease’s earliest, most vulnerable stages.