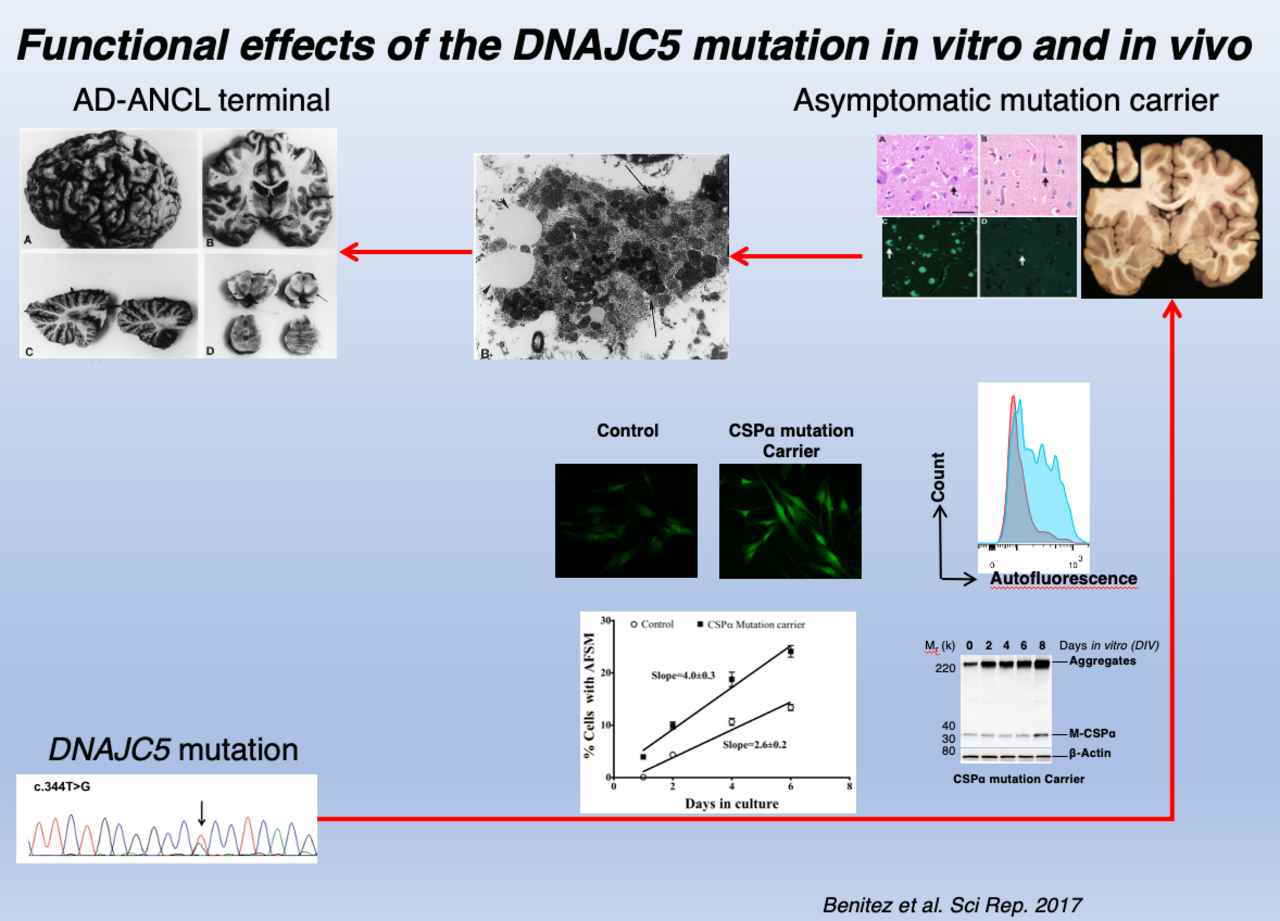
In 2011, we discovered that mutations in the DNAJC5 gene cause a neurodegenerative disease, the autosomal dominant form of neuronal ceroid lipofuscinosis (AD-ANCL). DNAJC5 encodes cysteine-string protein alpha (CSPα). In subsequent studies, we demonstrated in vitro and in vivo that CSPα plays a physiological role in the endo-lysosomes. In recent years, increasing in vitro evidence suggests that the misfolding-associated protein secretion (MAPS) pathway plays a role in the cell-to-cell transmission of prone-to-aggregate proteins such as α‐syn, tau, and amyloid. α-Syn and tau secretion seem to be primarily mediated by MAPS. CSPα is a crucial regulator of MAPS by mediating the cargo entry to the late endosomes. However, whether α-Syn or amyloid secretion and spreading in vivo are DNAJC5-dependent has yet to be determined. We study the role of variants in DNAJC5 on the risk of developing AD and PD using CRISPR/Cas9-corrected patient-derived induced pluripotent stem cells and primary cells to determine the role of DNAJC5 on the secretion and spreading of α-Syn and amyloid.