One in two women and one in four men over the age of 50 will suffer a fragility fracture in their remaining lifetime. These fractures lead to profound morbidity, increased mortality and high personal and societal costs. While effective treatments are available, they do not prevent all fractures and many women with osteoporosis remain untreated due to fears about rare side effects. Thus, new treatments for osteoporosis are needed, yet no new therapies have been approved since 2019.
Current FDA guidance requires fractures as the primary endpoint for clinical trials of new osteoporosis treatments, leading to

large, long and expensive trials. To address this, our team initiated the Study to Advance BMD as a Regulatory Endpoint (SABRE), with the goal of identifying a surrogate endpoint for fractures in future clinical trials. Initiated by the Foundation for the NIH in 2013, in a public-private partnership we were able to obtain individual patient data from all of the randomized clinical trials of osteoporosis medications (52 trials, over 160,000 individual subjects). We used this unique database to show that treatment-related changes in bone mineral density (BMD), assessed by standard dual-energy X-ray absorptiometry (DXA) scans, could predict the anti-fracture efficacy of osteoporosis therapies. We have since applied for formal qualification of BMD as a surrogate endpoint for fractures in future trials of new osteoporosis therapies via the FDA’s Biomarker Qualification Program. Our full qualification package is currently under review at the FDA, and we expect a ruling in the very near future. We anticipate that the landmark decision to approve BMD as a surrogate endpoint will accelerate innovation in osteoporosis treatments ultimately reducing the personal and societal burden of this all too common disease.
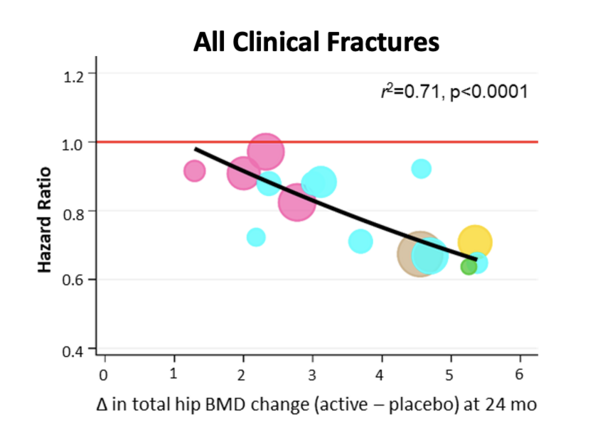
Meta-regression models showing a strong association between
total hip BMD changes measured at 24 months of treatment and
reduced fracture risk
Recent Publications
Schini, M., Lui, L. Y., Vilaca, T., Ewing, S. K., Thompson, A., Bauer, D. C., ... & Eastell, R. (2025). The relationship between baseline bone mineral density and fracture incidence in the placebo groups of randomized controlled trials using individual patient data from the FNIH-ASBMR-SABRE project. Journal of Bone and Mineral Research, 40(3), 307-314.
Vilaca, T., Schini, M., Lui, L. Y., Ewing, S. K., Thompson, A. R., Vittinghoff, E., ... & Bouxsein, M. L. (2024). The relationship between treatment-related changes in total hip BMD measured after 12, 18, and 24 mo and fracture risk reduction in osteoporosis clinical trials: the FNIH-ASBMR-SABRE project. Journal of Bone and Mineral Research, 39(10), 1434-1442.
Black, D. M., Thompson, A. R., Eastell, R., & Bouxsein, M. L. (2024). Bone mineral density as a surrogate endpoint for fracture risk reduction in clinical trials of osteoporosis therapies: an update on SABRE. The Lancet Diabetes & Endocrinology, 12(6), 371-373.
Schini, M., Vilaca, T., Lui, L. Y., Ewing, S. K., Thompson, A., Vittinghoff, E., ... & Eastell, R. (2024). Pre-treatment bone mineral density and the benefit of pharmacologic treatment on fracture risk and BMD change: analysis from the FNIH-ASBMR SABRE project. Journal of Bone and Mineral Research, 39(7), 867-876.
