Resources
Statistical Support
Alexander Brook, PhD, is the biostatistician working for the Department of Radiology at BIDMC.
Dr. Brook can assist you with the following:
- Study design
- Sample size determination/power analysis
- Survey/questionnaire design
- Data collection strategies
- Analysis and visualization
Please involve him in the design of your project as soon as feasible. The best way to do so is to email him at abrook@bidmc.harvard.edu with a short description of your project and your availability for an initial meeting.
Please review the relevant guides at https://bit.ly/StatsTips to avoid some of the more common mistakes in design and data collection.
Dr. Brook will make an effort to accommodate urgent requests for analysis for conference submissions, etc., but it is best to avoid these. If a short deadline cannot be avoided, please give him a heads-up as early as you are aware of the situation to allow him to plan his other obligations accordingly.
IRB Support
Contact Marc McCall at mmccall1@bidmc.harvard.edu. Marc will help submit all studies (including exempt, expedited, and full board) via iREX.
Exempt studies: Please respond to these 14 questions and email them to Marc. He will use this information to fill out the application form in iREX. He will reach out with questions as necessary. When completed, Marc will notify the PI, and the PI will need to log in, review, and sign the submission.
Expedited studies: The PI or their designee will need to fill in part B (and consent, questionnaires, surveys, and/or recruitment materials, if applicable) and email it to Marc. Marc will use that information to submit the application in iREX consisting of the following: Part A, Part M, Part P, Staffing form, Scientific Review form, and HIPAA waiver. If the study requires consent, questionnaires, surveys, and/or recruitment materials, these will be completed by the study PI and sent to Marc for submission.
The IRB may request additional information. COI (conflict of interest) declaration requests will be sent automatically to all staff upon submission. Once approved, the IRB will send a letter of activation so that the study may begin. Marc will retain all study-related documents on the research drive.
CITI (Collaborative Institutional Training Initiative) research training is mandatory. To begin the CITI training, visit https://about.citiprogram.org/ and create an account. Complete the Required Training “Basic Human Subjects Training - Basic Human Subjects Protection” (ID 23165).
Exempt Protocol Information – 14 questions
- Provide a brief summary of the research, including the background, rationale, and purpose.
- Describe the subject population to be studied. Include a description of how many subjects will be included and the inclusion and exclusion criteria.
- Describe any recruitment process, including advertisements to be used in the study.
- Describe the procedures and methods to be used in the study and the data to be collected (make sure you distinguish between those procedures done solely for the research and those that will be done outside the research context).
- Describe any compensation/reimbursement that will be provided to subjects.
- Are there any ethical concerns about the research or the individuals participating (invasion of privacy, undue influence to participate, and reputation of groups of individuals) based on research data?
- Describe how to maintain the privacy interests of participants (for example, when appropriate, discuss the study or collect information from participants in a location and/or in a manner that prevents others from knowing or hearing about the participants’ participation).
- Common language: "Data will be collected from the medical records. As the required data includes PHI, we will store the data on the hospital computer shared drive behind the firewall in a password-protected file. Access to the file will be restricted to PI and co-investigators."
- Describe how participants’ individually identifiable information will be recorded, ensuring that provisions are made to maintain the confidentiality of the data.
- Common language: "De-identified patient data will be stored on a secure server behind the BIDMC firewall. Only the PI and co-PIs will have access."
- If an activity involves interaction with participants (e.g., surveys, interviews, educational tests, benign behavioral intervention, etc.), describe the process by which participants will provide a prospective agreement to participate.
- If an activity involves interaction with participants (e.g., surveys, interviews, educational tests, benign behavioral intervention, etc.), will the prospective agreement be documented (e.g., participant signs agreement document)?
- If the study involves medical students, fellows, employees, or trainees, what procedure is in place to mitigate undue influence and maintain the privacy and confidentiality of their data?
- Is there a source of funding for this study?
- Please explain how this study can be done without funding.
- Staff list: include all people who will work on the project: PI, residents, fellows, students, etc.
Grant Writing
Dr. Deb Burstein, dburstei@bidmc.harvard.edu, has extensive experience with successful grant writing. We highly recommend seeking her advice early in the process of your grant writing, ideally at the stage of idea generation, to allow time for iterations in the process.
Data Extraction for Research
Our IT team may assist in extracting demographics, radiology reports, numerical data parsed from the reports, correlation with external identifier lists, and radiation dose data from Radiology Information Systems. Please contact Larry Barbaras with the following:
- IRB approval document
- Details of research query: dates, search terms, specific types of studies, parameters to match
- Details needed in the output: demographics, radiology reports, dose data, etc.
You can also query hospital information system medical records through Clinical Query 2 using billing codes. The data provided includes demographics, ICD-9 and ICD-10 diagnoses, medications, laboratory tests, procedures CPT-4 and ICD-9 and ICD-10, vital signs, and visits. The contact person is Griffin Weber or Academic Computing.
Additional information that is not included in CQ2 can be obtained through InSIGHT Core. Contact Karla Pollick.
Research PACS
Research PACS is a solution that can be used to collect and view imaging data as part of an approved project for research, quality improvement, or an education effort with a well-defined scope. Only data required for the project can be stored in Research PACS. Additionally, data in a Research PACS project should never be used to make a clinical decision for a patient. All data related to patient care must be documented in the clinical record, per standard clinical practice.
Sign into InfoRad on the Portal at https://inforad.bidmc.harvard.edu/ and complete the request form “Research PACS - Project Request Form (Beta)”.
Once you complete and submit the form, a Research PACS team member will be in touch to follow up regarding IRB approvals, project endorsements (for quality improvement projects), etc.
Note: You must complete the entire request form and click the "Submit" button for the request to be sent to the Research PACS Team.
Mini-Grant Applications
Mini-grants (up to 20k) are available for the faculty and trainees to support pilot studies, which will serve as a proof of concept and provide preliminary data for future external grant applications. The applications will be accepted throughout the year and reviewed by the Radiology Research Applications Committee.
Please discuss your application with Dr. Olga Brook and request an application form from Ema Pinjic.
Research Interns and Fellows
If you are seeking a position as a research fellow or intern in the Department of Radiology at BIDMC, please email an individual PI or Ema Pinjic at epinjic@bidmc.harvard.edu.
A research fellow or intern is someone with a graduate degree who, as a junior researcher, performs academic research under the guidance and supervision of faculty PIs.
A research fellow is a paid position that should be paid according to NIH standards for their respective PGY rank.
A research intern is an unpaid position available to someone with no prior experience with research. This position can only be used for 12 months.
Information for faculty:
To onboard a new Radiology Research Fellow or Research Student, please contact Ema Pinjic, Associate Director of Radiology Research, at epinjic@bidmc.harvard.edu.
In the initial email, please send the following:
- The fellow’s or intern’s CV
- Start date
- Whether the position is paid or unpaid
- Fund source and number are needed for all intake forms, even when a fellow/student is unpaid
- Need for a visa
- English proficiency assessment
- Intended workplace – in the hospital or remote
Space and number of workstations is limited in the Department. If a fellow/intern needs to work in the hospital, they may need to share a workstation with another research fellow/intern on a predetermined schedule.
The Associate Director will help with the completion of the mandatory forms: the Research Fellow Intake Form* or Research Student Intake Form, Candidate Interview Evaluation Form with Score – for all candidates, Attestation of Outside Support Form – for unpaid fellows, Certification of English Language Proficiency Form – for fellows and students requiring a visa.
The PI will need to review and sign the forms. The Associate Director will review the completed and signed forms and will send them to Edward Nhan, Research Administrator (RA), at enhan@bidmc.harvard.edu.
The RA will review all information for accuracy/completeness before approving and providing the Cost Center number. The RA can also provide insight into the suggested pay scale for Research Fellows. The RA will send back documentation to the Associate Director.
For unpaid research interns who do not require a visa, documents will be sent to HR directly.
For research fellows, a job requisition must also be created in Workday. After all documents are approved, they will be submitted to Human Resources (HR) and/or the Immigration Specialist.
Onboarding timeline:
- HR takes about three weeks for the onboarding process. The total onboarding time is 5-6 weeks, if there is no need for a visa.
- If a visa is required, the Immigration process may take at least three months. Please start the process in advance to ensure the desired start date. Depending on what country the fellow is coming from, the immigration process could last much longer. On average, the total onboarding time for a fellow requiring a visa is 5-6 months.
Once onboard, the Associate Director of Research will help to ensure the completion of necessary onboarding paperwork, such as setting up information system access and assigning required myPATH trainings.
The latest information, forms, and documents about the Research Fellow onboarding process are on the BIDMC Portal at https://portal.bidmc.org/-/media/Files/Intranets/Research/ResFellows/PRFGuidelines.ashx.
The salary amount for a research fellow is according to the career level per NIH NRSA guidelines, 2022/2023.
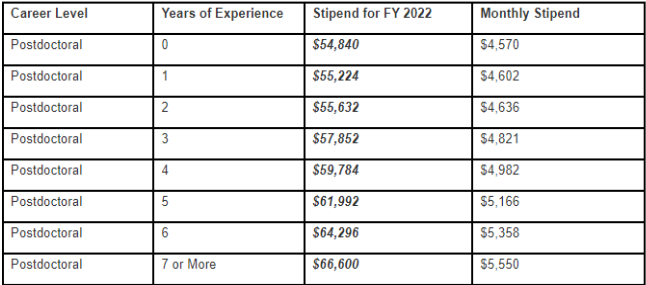
Please incorporate a fringe rate of 30% into your budget proposals.

Research Projects Log
Trainees are required to log their scholarly and PQI projects in the following database.
