Leucine-rich repeat kinase 2 (LRRK2) is a critical protein associated with the pathogenesis of Parkinson's disease (PD), particularly through its influence on the endolysosomal system and the clearance of alpha-synuclein (aSyn) aggregates. The G2019S mutation in LRRK2 is the most prevalent pathogenic variant, leading to increased kinase activity that disrupts the function of Rab GTPases, which are essential for vesicular trafficking and lysosomal dynamics (Lis et al., 2018; Ho et al., 2019). This dysregulation results in impaired endosomal and lysosomal function, contributing to neurodegeneration and the accumulation of alpha-synuclein aggregates, which are characteristic of PD pathology (Eguchi et al., 2018; Jeong et al., 2018).
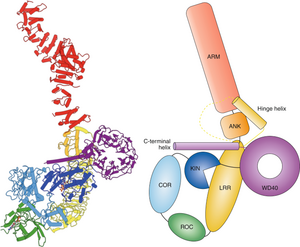
In vitro and in vivo studies show that the G2019S mutation exacerbates the accumulation of aSyn due to compromised autophagy and lysosomal dysfunction (Lis et al., 2018; Eguchi et al., 2018). Specifically, LRRK2 phosphorylates Rab proteins, which inhibits their release from membranes, leading to their accumulation and dysfunction (Ho et al., 2019; Jeong et al., 2018).
In contrast, the VPS35 D620N mutation affects the endolysosomal system through a different mechanism. VPS35 is part of the retromer complex, which recycles proteins from endosomes. The D620N mutation enhances LRRK2-mediated Rab phosphorylation, indicating a synergistic effect that exacerbates endolysosomal stress (Rinaldi et al., 2022; Mir et al., 2018).
