Clinical electrical stimulation systems are increasingly common therapeutic options to treat a broad range of medical conditions, such as cardioverter-defibrillators, pacemakers, spinal cord stimulators, and deep brain stimulators. Despite their remarkable success, a significant limitation of these medical devices is their limited compatibility with magnetic resonance imaging (MRI), a standard and widely used diagnostic tool in medicine. A primary concern when performing MRI examinations in patients with electrically conductive implants is the antenna effect, which can potentially cause a large amount of energy to be absorbed in the tissue, leading to heat-related severe injury.
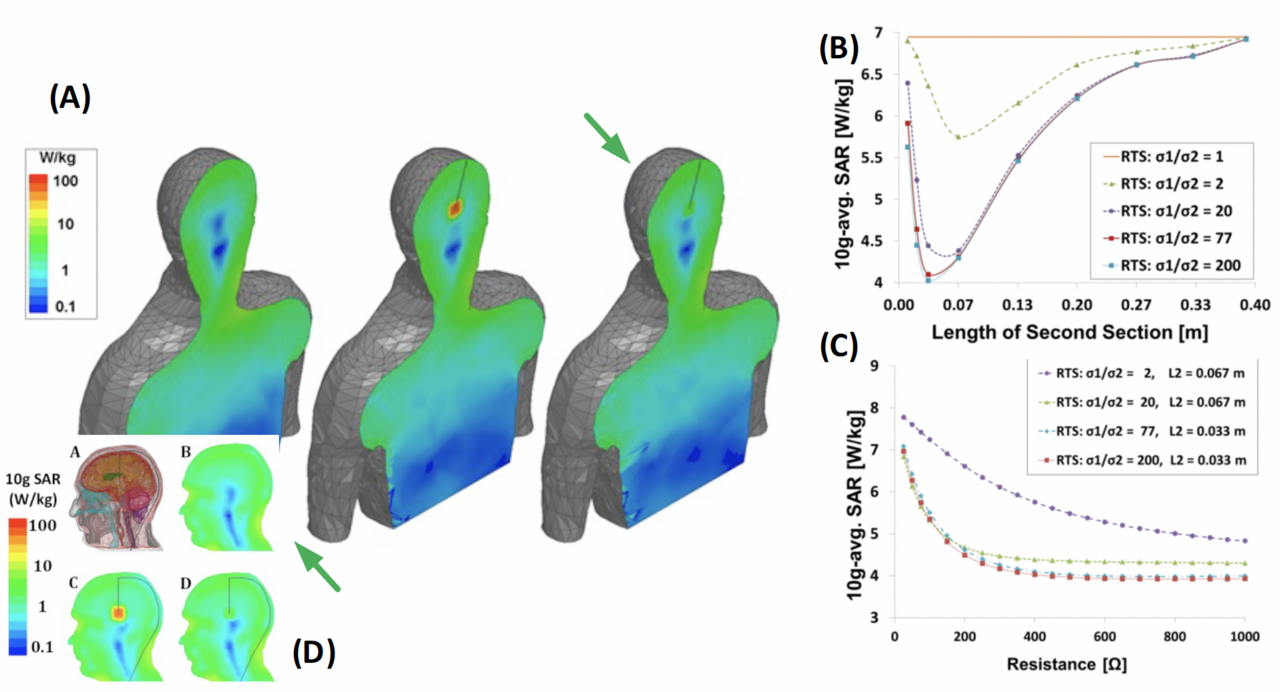
In this NIH-funded (R01NS128962) collaboration with Giorgio Bonmassar and Ilknur Ay, we are designing, developing, and testing a novel metamaterials technology to produce MRI conditional leads that could be used in implanted electrical recording and stimulation devices. The innovative nanoscale thin-film metamaterial is truly the only MRI cloaking technology that does not occupy any "brain" space compared to additional RF-choking components. We are developing a general framework for any arbitrary electrical stimulation lead implanted in the body to prevent a build-up of induced currents and reduce imaging artifacts during a 3 Tesla MRI. Furthermore, we are developing novel electrocorticography (ECoG) electrodes based on biocompatible materials that will be stretchable, and conformable for optimal biocompatibility, safety, and performance. These novel electrodes will be thin and flexible and can be created in a wide range of configurations (i.e., strips, grids, and various combinations) for different applications. Notably, the electrodes will be MRI-safe and CT artifact-free, allowing for perfect registration of the electrode location to the brain anatomy. The electrodes also permit the combination of intracranial (depth and cortical) recordings with fMRI imaging, leading to a greater understanding of the neural organization in both individual patients and for neuroscientific knowledge.
