High density electrocorticography has shown promise as a diagnostic and prognostication tool in specific neurological conditions. Existing approaches to decoding motor, sensory, and speech signals for brain-computer interface currently rely on invasive electrodes that penetrate the pia matter. This leads to gliosis and signal loss over time. Thin-film electrodes, which are placed on the brain surface and do not penetrate the cortex, offer improvements over these penetrating electrodes. However, conventional 'macro'-ECoG electrode arrays used currently in intraoperative monitoring are predominantly handmade and therefore restricted in feature sizes, typically with an electrode diameter of several mm and an inter-contact distance on the order of 1 cm.
Currently available high-density eletrode arrays have been around for over a decade and involve penetrating the nervous system with rigid microelectrodes. For example, the FDA-cleared Utah Electrode Array has been the gold-standard electrode for monitoring neural activity in the human brain. Thin-film-technology involves printing a layer of microelectrodes on a flexible thin polymer that allows higher density of electrodes, no penetration of the brain, and access to large cortical surface areas, addressing many of the shortcomings of existing electrode technology. For reference, Figure 1 below shows the differentiating characteristics between the existing Utah 96 micro-electrode array and the Layer 7 surface cortical micro-electrode array.
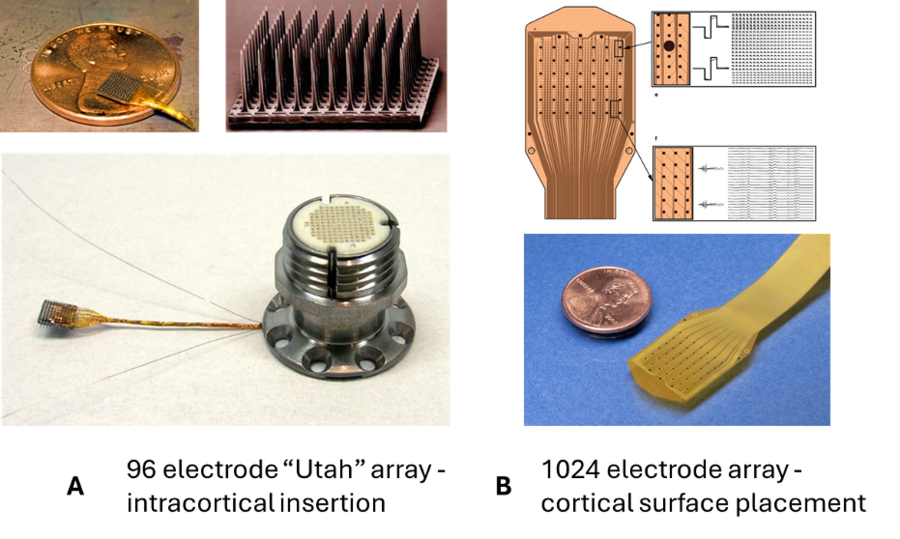
There has been growing interest in novel fabrication techniques allowing for the creation of a new generation of micro-ECoG arrays. In practice, these arrays provide superior performance compared to standard arrays, with early studies showing they reliably reveal cortical somatosensory response patterns which would not have been resolvable with conventional ECoG, more accurately predict spikes and discriminate phonemes, and differentiate between ictal and interictal patterns that would show up as nearly identical spikes at the resolution of clinical EEG.
The ability to capture neural patterns associated with fine motor function, sensation, and speech is dependent on signal quality and high channel count. We are collaborating with Precision Neuroscience to investigate whether the Layer 7 Cortical Array can reliably record neural signals over several days and whether these signals are sufficient to decode function for potential brain-computer interface applications.
We are implanting these high resolution ECoG arrays to detect neural patterns or cortical events which may be correlated to volitional movement, speech, and sensation. This includes determining electrical signal characteristics from cortical regions of interest (i.e., sensory, motor or language regions), as well as correlating that neural activity with behavioral tasks. Information gained will be used to investigate high temporal and spatial representation of neural signals. This will also provide valuable information as to how the brain and its regions work together to control movement, language or interpret sensory stimulus; for refining decoding algorithms for brain–computer interface functionality.
