Jack Lawler PhD
Vice-Chair for Research and Professor of Pathology
Beth Israel Deaconess Medical Center and Harvard Medical School
Department of Pathology
Office: RN-270D
Phone: 339-440-0787
Administrative Assistant: Lydia Moss

Research Areas
Thrombo-spondin Function
The thrombospondins (TSPs) are extracellular, calcium-binding proteins that regulate cellular phenotype by orchestrating the assembly of multi-protein complexes on the cell surface. These complexes include CD36, vascular endothelial growth factor receptor 2 (VEGFR2), transforming growth factor beta, proteoglycans, integrins, and integrin- associated proteins. TSP-1 on the cell surface inhibits (1) proliferation of endothelial and tumor cells, (2) angiogenesis, and (3) the growth of experimental and naturally occurring tumors. The lab is currently elucidating the role of TSP-1 in tumor progression and metastasis.
Thrombo-spondin Structure
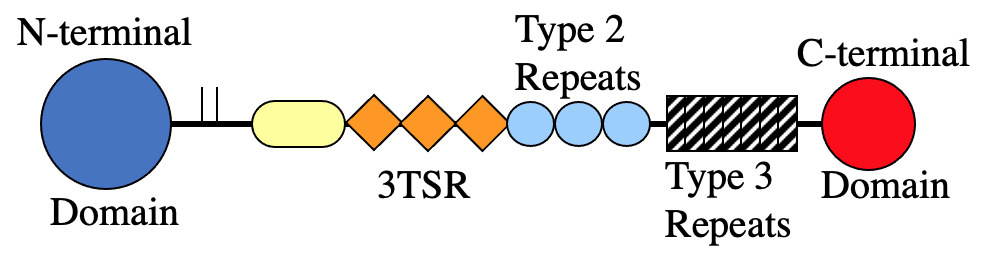
Multiple structural domains comprise the TSP proteins. Some of these domains are common in large extracellular matrix proteins and probably reflect exon shuffling during evolution. For example, the TSP type 2 repeats are EGF repeats. Other domains are unique to the TSPs. The last type 2 repeat, the type 3 repeats, and the C-terminal domain comprise the "signature domain" of the TSPs because this portion of the molecules is highly conserved.
Clinical Development of Fc3TSR
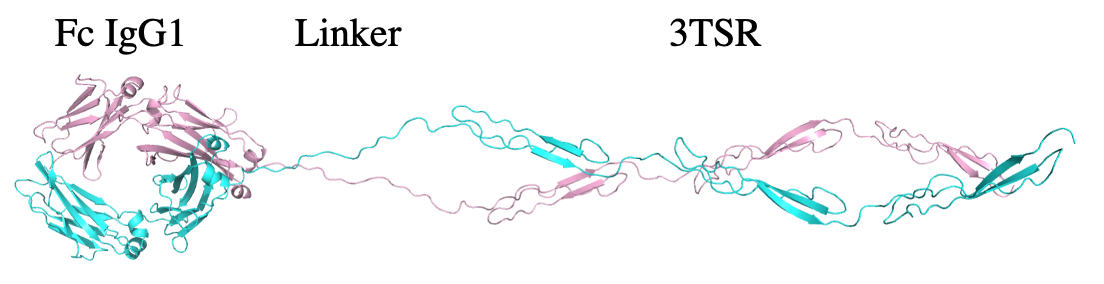 Fc3TSR is an IgG1 fusion protein that contains 2 copies of the 3 type 1 repeats of TSP-1. The type 1 repeats were first identified in TSP-1 and were later found to be a novel protein fold by X-ray crystallography. Several of these proteins are involved in axon guidance and inhibition of angiogenesis. Fc3TSR is under development as a potent inhibitor of ovarian and pancreatic cancer as a single agent and in combination with chemotherapy and immunotherapy.
Fc3TSR is an IgG1 fusion protein that contains 2 copies of the 3 type 1 repeats of TSP-1. The type 1 repeats were first identified in TSP-1 and were later found to be a novel protein fold by X-ray crystallography. Several of these proteins are involved in axon guidance and inhibition of angiogenesis. Fc3TSR is under development as a potent inhibitor of ovarian and pancreatic cancer as a single agent and in combination with chemotherapy and immunotherapy.