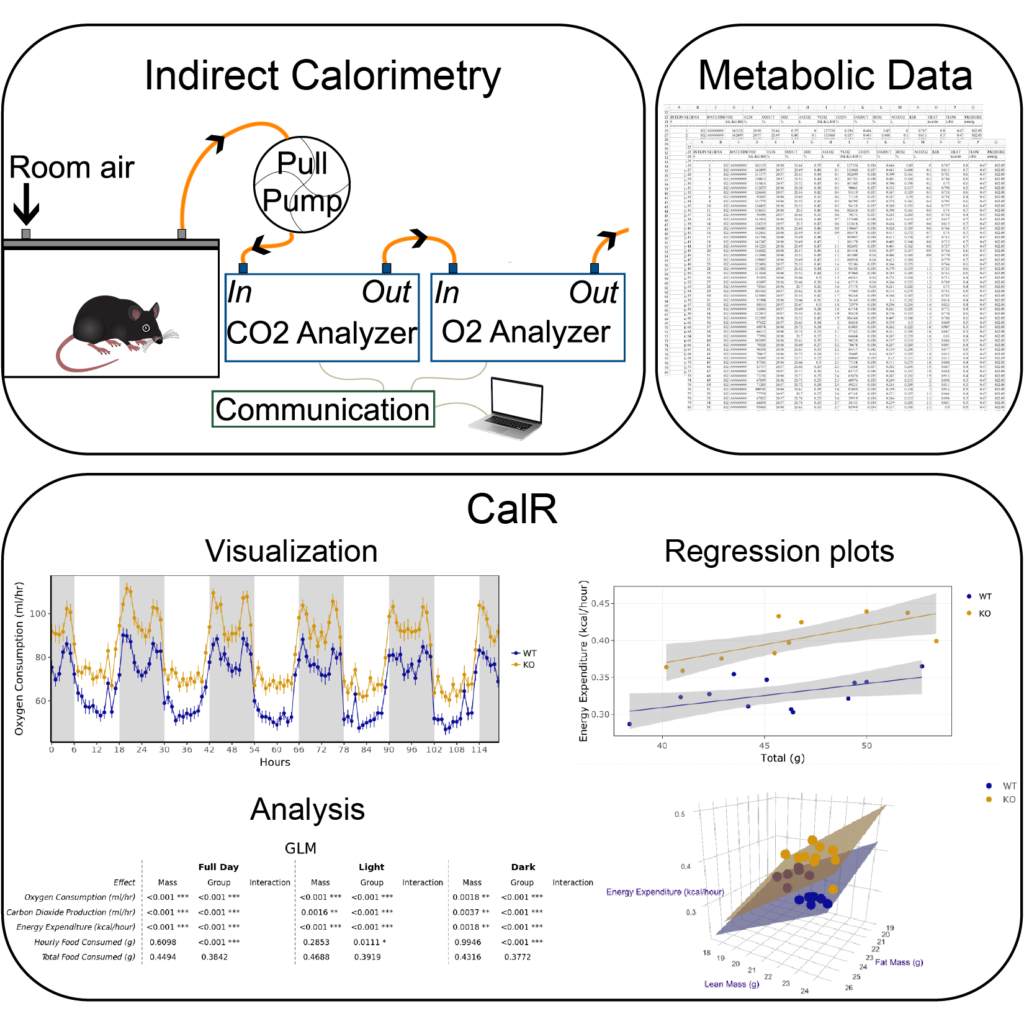
We use indirect calorimetry systems to measure metabolic rates, physical activity, and food intake in laboratory animals. These components impact energy balance and body weight. These indirect calorimetry systems generate a flood of raw data that requires time-consuming manual manipulation for formatting, data cleaning, quality control, and visualization. Beyond data handling, analysis of indirect calorimetry experiments requires specialized statistical treatment to account for differential contributions of fat mass and lean mass to metabolic rates. Surprisingly, we found no tools or resources exist to help address these shortcomings. To address this problem, we developed a free online tool, CalR, that helps scientists quickly and efficiently analyze indirect calorimetry data by providing standardized methods for reproducible research and a site to store and aggregate datasets (Cell Metabolism 2018). The pilot version of CalR we launched is a user-friendly and sophisticated web tool that uses a graphical user interface to import data files from different instruments (CLAMS, TSE, and Sable), quickly visualize experimental results, and perform basic statistical analyses. CalR has now been used to run analysis on more than 90,000 experiments. The broad goal of this research is the development of a framework that delivers modern tools for the analysis of the physiological data affecting body weight. Using these tools, we have been able to perform analysis of studies of data from over 30,000 mice (eLife 2020). We enjoyed many productive collaborative efforts to study the effects of genes, environmental stimuli, or pharmacological agents on metabolic physiology.